Pyridopyrroloquinoxaline
A substituted pyridopyrroloquinoxaline, or more specifically a substituted octahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline, also known as a substituted heterocycle fused γ-carboline, is a further-cyclized and substituted tetracyclic derivative of the tricyclic alkaloid γ-carboline as well as an analogue of the atypical antipsychotic lumateperone.[1][2][3][4][5] They can additionally be thought of as analogues of cyclized tryptamines like the β-carbolines or harmala alkaloids such as harmaline, but are not technically tryptamines themselves.
Pyridopyrroloquinoxalines are notable for their varying interactions with the serotonin 5-HT2A receptor as well as with other monoamine receptors.[1][6] Lumateperone and deulumateperone are serotonin 5-HT2A receptor antagonists with antipsychotic properties, IHCH-7113 is a putatively psychedelic serotonin 5-HT2A receptor full agonist with a robust head-twitch response in rodents, and IHCH-7086, IHCH-7079, and ITI-1549 are putatively non-hallucinogenic β-arrestin-biased serotonin 5-HT2A receptor partial agonists with psychoplastogenic and/or antidepressant-like effects in preclinical studies.[7][1][8][9][10][11] The broad receptor interactions of some of these compounds have been studied.[6][1]
Pyridopyrroloquinoxalines with serotonin 5-HT2A receptor agonistic activity such as IHCH-7113 and IHCH-7086 were first described in the scientific literature by Dongmei Cao and colleagues by 2022.[1] As of 2025, ITI-1549 is under development by Intra-Cellular Therapies for the treatment of mood and other psychiatric disorders.[11]
List of pyridopyrroloquinoxalines
| Structure | Name | Chemical name |
|---|---|---|
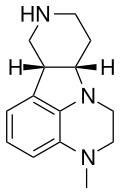
 | IHCH-7113 | (6bR,10aS)-3-methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline |
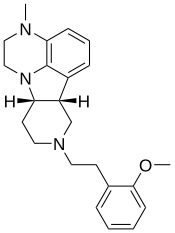 | IHCH-7079 | (6bR,10aS)-2,3,6b,7,8,9,10,10a-octahydro-8-[2-(2-methoxyphenyl)ethyl]-3-methyl-1H-pyrido[3′,4′:4,5]pyrrolo[1,2,3-de]quinoxaline |
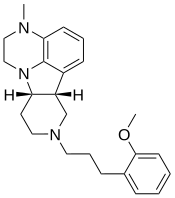 | IHCH-7086 | (6bR,10aS)-2,3,6b,7,8,9,10,10a-octahydro-8-[3-(2-methoxyphenyl)propyl]-3-methyl-1H-pyrido[3′,4′:4,5]pyrrolo[1,2,3-de]quinoxaline |
| Lumateperone (ITI-007) | 1-(4-fluorophenyl)-4-(3-methyl-2,3,6b,9,10,10a-hexahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalin-8(7H)-yl)-1-butanone | |
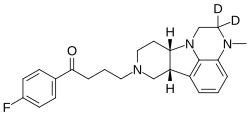 | Deulumateperone (ITI-1284) | 1-(4-fluorophenyl)-4-[(6bR,10aS)-3-methyl-2,3,6b,9,10,10a-(2-2H)hexahydro(2-2H)-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalin-8(7H)-yl]butan-1-one |
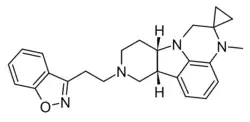 | ITI-1549 | ? |
Other known pyridopyrroloquinoxalines include IHCH-7081, IHCH-7087, IHCH-7088, IHCH-7089, IHCH-7112, and IHCH-7120.[1]
Related compounds
| Structure | Name | Chemical name |
|---|---|---|
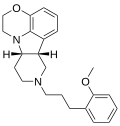 | IHCH-8134 | (6bR,10aS)-8-(3-(2-methoxyphenyl)propyl)-1,2,6b,7,8,9,10,10a-octahydro-[1,4]oxazino[2,3,4-hi]pyrido[4,3-b]indole |
See also
- Cyclized tryptamine
- Substituted β-carboline
- Ibogalog
- Substituted lysergamide
- Non-hallucinogenic 5-HT2A receptor agonist
References
- 1 2 3 4 5 6 Cao D, Yu J, Wang H, Luo Z, Liu X, He L, Qi J, Fan L, Tang L, Chen Z, Li J, Cheng J, Wang S (January 2022). "Structure-based discovery of nonhallucinogenic psychedelic analogs". Science. 375 (6579): 403–411. Bibcode:2022Sci...375..403C. doi:10.1126/science.abl8615. PMID 35084960.
- ↑ "Pyridopyrroloquinoxaline compounds, their compositions and uses in therapy". Google Patents. 20 July 2018. Retrieved 29 July 2025.
- ↑ "Lumateperone and derivatives thereof for modulating the nervous system". Google Patents. 16 February 2024. Retrieved 29 July 2025.
- ↑ "A pyridopyrroloquinoxaline compound and its medical use". Google Patents. 20 December 2019. Retrieved 29 July 2025.
- ↑ Dai J, Dan W, Zhang Y, Wang J (September 2018). "Recent developments on synthesis and biological activities of γ-carboline". Eur J Med Chem. 157: 447–461. doi:10.1016/j.ejmech.2018.08.015. PMID 30103193.
- 1 2 Sharp T, Ippolito A (May 2025). "Neuropsychopharmacology of hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists". Br J Pharmacol bph.70050. doi:10.1111/bph.70050. PMID 40405723.
- ↑ Duan W, Cao D, Wang S, Cheng J (January 2024). "Serotonin 2A Receptor (5-HT2AR) Agonists: Psychedelics and Non-Hallucinogenic Analogues as Emerging Antidepressants". Chem Rev. 124 (1): 124–163. doi:10.1021/acs.chemrev.3c00375. PMID 38033123.
Truncation of the tail group attached to the tetracyclic core of lumateperone resulted in compound IHCH-7113 (166, Figure 14C), which turned out to be a potent 5-HT2AR agonist (Gq BRET, EC50 = 58.9 nM (99.2%); β-arrestin2 BRET, EC50 = 44.7 nM (95.6%)). The introduction of a 2-methoxyphenyl tail bridged by either two or three CH2 groups led to compounds IHCH-7079 (167) and IHCH-7086 (168) (Figure 14C), respectively. Both compounds bind to the 5-HT2AR with high affinity (Ki = 16.98 and 12.59 nM, respectively; [3H]-LSD). In functional assays, compound IHCH-7079 showed a preference for β-arrestin2 recruitment (bias factor = 54.58). Compound IHCH-7086 behaved as a weak partial agonist in the βarrestin2 BRET assay (EC50 = 204.2 nM, Emax = 12.7%) but exhibited no Gq activation. Therefore, both compounds are β-arrestin-biased. Neither IHCH-7079 nor IHCH-7086 induced HTRs in mice at a dose of 10 mg/kg, but both compounds demonstrated significant antidepressant effects in acute stressand corticosterone-induced depression-like mouse models.60 Our results suggest that β-arrestin-biased 5-HT2AR partial agonists hold the potential as nonhallucinogenic psychedelic analogues for the development of novel antidepressant drugs. [...] Our research has identified β-arrestin-biased 5-HT2AR agonists, such as compounds IHCH-7079 and IHCH-7086,60 which exhibit varying degrees of β-arrestin-bias (Table 1). Notably, IHCH-7086 acts as a completely β-arrestin-biased 5-HT2AR agonist, with a high binding affinity at the receptor but no activation of the Gq pathway. We demonstrated that IHCH-7086 produces significant antidepressant effects in the absence of hallucinogenic effects in mouse behavioral models.60 [...]
- ↑ Yin YN, Gao TM (January 2023). "Non-hallucinogenic Psychedelic Analog Design: A Promising Direction for Depression Treatment". Neurosci Bull. 39 (1): 170–172. doi:10.1007/s12264-022-00933-7. PMC 9849505. PMID 35927548.
- ↑ Ippolito A, Vasudevan S, Hurley S, Gilmour G, Westhorpe F, Churchill G, Sharp T (June 2025). "Evidence that 5-HT2A receptor signalling efficacy and not biased agonism differentiates serotonergic psychedelic from non-psychedelic drugs". Br J Pharmacol bph.70109. doi:10.1111/bph.70109. PMID 40545270.
- ↑ Atiq MA, Baker MR, Voort JL, Vargas MV, Choi DS (July 2025). "Disentangling the acute subjective effects of classic psychedelics from their enduring therapeutic properties". Psychopharmacology (Berl). 242 (7): 1481–1506. doi:10.1007/s00213-024-06599-5. PMC 12226698. PMID 38743110.
What followed was the development of ß-arrestin-biased ligands ('IHCH-7079' and 'IHCH-7086') without detectable Gq activity. Upon testing for hallucinogenic activity, IHCH-7079 and IHCH-7086 failed to produce any HTR and were also found to abolish LSD-induced HTR. In vivo studies of antidepressant potential, as measured by the FST and tail suspension test (TST), revealed significantly attenuated acute-restraint stress-induced immobility with the two ligands, an efect that was eliminated by a selective 5-HT2AR antagonist (MDL100907).
- 1 2 Dutheil, Sophie; Lehmann, Vanessa E.; Awadallah, Nora; John, Neelu; Zhang, Lei; Yao, Wei; Li, Peng; Snyder, Gretchen; Davis, Robert (2025). "319. Discovery and Development of ITI-1549: A Novel Serotonin 5-HT2A Agonist, Non-Hallucinogenic Neuroplastogen, for the Treatment of Neuropsychiatric Disorders". Biological Psychiatry. 97 (9): S227. doi:10.1016/j.biopsych.2025.02.557. Retrieved 29 July 2025.