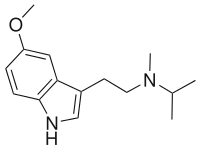
5-MeO-MiPT
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 5-Methoxy-N-methyl-N-isopropyltryptamine; Moxy; MSD-001; MSD001 |
| Routes of administration | Oral, smoking[1] |
| Drug class | Non-selective serotonin receptor agonist; Serotonin 5-HT2A receptor agonist; Serotonergic psychedelic; Hallucinogen; Entactogen |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Onset of action | ~15–45 minutes[1] |
| Duration of action | 4–6 hours[1] or 3–8 hours[3][4] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.223.426 |
| Chemical and physical data | |
| Formula | C15H22N2O |
| Molar mass | 246.354 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
5-MeO-MiPT, also known as 5-methoxy-N-methyl-N-isopropyltryptamine or by its nickname Moxy, is an atypical psychedelic drug of the tryptamine and 5-methoxytryptamine families.[1][3] It has unique and unusual effects compared to other psychedelic tryptamines.[1][3][5] At low doses, its effects include stimulation, tactile and sexual enhancement, some MDMA-like entactogenic effects, and introspective and mild perceptual changes with little or no psychedelic visuals or time dilation, whereas at higher doses, it produces 5-MeO-DMT-like classical psychedelic effects.[1][3][5] It is usually taken orally or smoked.[1]
The drug acts as a non-selective serotonin receptor agonist, including of the serotonin 5-HT1A, 5-HT2A, and 5-HT2C receptors, among others.[6][7][8] It is closely related in chemical structure and effects to 5-MeO-DiPT, and is also related to other tryptamines like 5-MeO-DMT, 4-HO-MiPT, and MiPT.[1][3][5]
5-MeO-MiPT was first described in the literature by Alexander Shulgin and David Repke and colleagues in 1985.[3][9] It was later described by Shulgin in greater detail in his 1997 book TiHKAL (Tryptamines I Have Known and Loved).[1] The United States Drug Enforcement Administration (DEA) unsuccessfully tried to ban 5-MeO-MiPT in the 2020s.[3] This effort was opposed by the psychedelic community.[3]
Use and effects

In his book TiHKAL (Tryptamines I Have Known and Loved), Alexander Shulgin lists the dose of 5-MeO-MiPT as 4 to 6 mg orally and 12 to 20 mg smoked.[1] A wider recreational dose range of 0.5 to 20 mg or more orally has also been reported however.[10][3][4] Oral doses of 1 to 3 mg have been described as light, 3 to 8 mg as common or moderate, and 8 to 12 mg as strong.[4] Its onset of action when taken orally is described as very rapid, occurring within 15 to 45 minutes, peak effects appear to occur after around 1 to 2 hours, and its duration as 4 to 6 hours.[1] However, other sources state its duration as 3 to 8 hours.[3][4]
5-MeO-MiPT has been described as having unique and unusual effects relative to other psychedelic tryptamines.[1][3][5] The effects of 5-MeO-MiPT differ depending on whether it is taken orally or smoked and are highly dose-dependent.[1][3] When taken orally at relatively low doses like 4 to 6 mg, it is usually described as not producing psychedelic visuals or related sensory effects and as producing only hints of time dilation.[1][3] However, it is said to produce a stoned state that includes an ease of interpretive fantasy, dream-like perception, intense conceptual thought and philosophical thinking, and mild perceptual effects like altered depth perception, minor wave pattern in peripheral vision, and slightly enhanced auditory acuity.[1] Moreover, the drug is described as producing stimulation, greatly enhanced tactile sensation and eroticism, enhanced music appreciation, tingling, shakes, and mild motor impairment.[1][3] Its head space is described as relatively "shallow", less confusing, and more easily tolerated compared to classical psychedelics.[3] Its effects have been described by users variably as both pleasant and negative.[1][3]
When smoked, 5-MeO-MiPT is described as having effects similar in many regards to those of 5-MeO-DMT.[1] These effects of smoked 5-MeO-MiPT include a powerful rush (but less intense than 5-MeO-DMT), loss of coherent thought, not much in the way of visuals, closed-eye visuals of moving and colored geometric patterns, intense waves of mental imagery of emotionally infused memories, impressive recall of early memories, intense depersonalization or disorientation of the normal sense of being a person in a body, loss of immediate contact with surroundings, emotional lability including laughing, crying, and vocal outbursts, groaning, writhing, shaking around, and general disorientation.[1] It is described as having a very rapid onset, with the peak phase lasting less than 30 minutes and waves continuing for up to a few hours.[1] It was described by one user as feeling like a hybrid between diethyltryptamine (DET) and 5-MeO-DMT.[1]
5-MeO-MiPT is also known by its nickname "Moxy"[3] and is closely related both in terms of chemical structure and effects to 5-MeO-DiPT (also known as "Foxy Methoxy").[5][1] These two serotonergic tryptamines at low doses have been described as very aphrodisiac and much more stimulant-like and party drugs than classical psychedelics.[5] However, they have been described as not innately aphrodisiac, but instead as enhancing tactile sensation in a way that lends itself to sex.[5] Matthew Baggott has described 5-MeO-MiPT as having some MDMA-like entactogenic effects at low doses, including tactile enhancement and feelings of empathy, intimacy, and closeness with others, and as producing classical psychedelic effects at higher doses.[11]
In addition to its use on its own, 5-MeO-MiPT, along with the related tryptamine psychedelic 4-HO-MET, is employed as a component of the MDMA-mimicking Borax combo.[3][12][13][14]
Side effects
Adverse effects of 5-MeO-MiPT include loss of appetite and insomnia.[1] Low-dose 5-MeO-MiPT did not cause any serious histopathological effects on the liver, kidney, and brain. High doses induce apoptotic cell death through caspase activity especially in some parts of the organs.[15] There is no known documentation of death attributed to the use of 5-MeO-MiPT alone.[3]
Interactions
Pharmacology
| Target | Affinity (Ki, nM) |
|---|---|
| 5-HT1A | 12–143 (Ki) 610–>10,000 (EC50) 109% (Emax) |
| 5-HT1B | 303 |
| 5-HT1D | 23 |
| 5-HT1E | 3,496 |
| 5-HT1F | ND |
| 5-HT2A | 113–449 (Ki) 5.9–566 (EC50) 82–101% (Emax) |
| 5-HT2B | 59 (Ki) 1,500 (EC50) 12% (Emax) |
| 5-HT2C | 790–2,186 (Ki) 179 (EC50) 101% (Emax) |
| 5-HT3 | >10,000 |
| 5-HT4 | ND |
| 5-HT5A | 953 |
| 5-HT6 | 130 |
| 5-HT7 | 20 |
| α1A | >12,000 |
| α1B | >10,000 |
| α2A | 175–5,300 |
| α2B | 1,693 |
| α2C | 637 |
| β1–β2 | >10,000 |
| D1 | >25,000 |
| D2 | >25,000 |
| D3 | 2,470–>25,000 |
| D4 | 6,331 |
| D5 | >10,000 |
| H1 | 3,900–4,819 |
| H2–H4 | >10,000 |
| I1 | 879 |
| TAAR1 | >15,000 (rat/mouse) |
| σ1 | >10,000 |
| σ2 | 918 |
| SERT | 3,300–6,409 (Ki) 2,680–29,768 (IC50) >100,000 (EC50) |
| NET | >22,000 (Ki) 84,000 (IC50) >100,000 (EC50) |
| DAT | >26,000 (Ki) >100,000 (IC50) >100,000 (EC50) |
| Notes: The smaller the value, the more avidly the drug binds to the site. Refs: [16][8][17][6][7][18] | |
Pharmacodynamics
The mechanism that produces the hallucinogenic and entheogenic effects of 5-MeO-MiPT is thought to result primarily from serotonin 5-HT2A receptor agonism, although additional mechanisms of action such as inhibition of monoamine oxidase (MAO) might also be involved.[9][16] In addition to the serotonin 5-HT2A receptor, 5-MeO-MiPT also potently binds to and/or activates other serotonin receptors, such as the serotonin 5-HT1A, 5-HT2B, and 5-HT2C receptors.[6]
In addition to the serotonin receptors, 5-MeO-MiPT has also been found to show significant affinity to the serotonin transporter (SERT) and norepinephrine transporter (NET), thereby acting as a moderately potent serotonin–norepinephrine reuptake inhibitor (SNRI).[8] However, subsequent research contradicted the preceding findings and found that 5-MeO-MiPT did not significantly bind to or inhibit the human monoamine transporters.[6] The drug is also inactive as a monoamine releasing agent.[16]
Chemistry
5-MeO-MiPT is in a class of compounds commonly known as tryptamines, and is the N-methyl-N-isopropyl homologue of 5-MeO-DMT. The full name of the chemical is 5-methoxy-N-methyl-N-isopropyltryptamine.
Analogues
Analogues of 5-MeO-MiPT include MiPT, 5-MeO-DMT, 5-MeO-DiPT, and 5-MeO-MET, among others.
Detection
5-MeO-MiPT causes the ehrlich reagent to turn purple then fade to faint blue. It causes the marquis reagent to go yellow through to black.[19]
Exposing compounds to the reagents gives a colour change which is indicative of the compound under test. The following test results are from protestkit.
| 5-MeO-MiPT | Marquis | Mecke | Mandelin | Liebermann | Ehrlich | Hofmann | Simon’s |
|---|---|---|---|---|---|---|---|
| Freebase | Orange to brown | Orange red | Deep greenish brown | Unknown | Purple | No reaction | No reaction |
| HCl | Orange to brown | Red to brown | Greenish brown | Brown | Violet to purple | Green | Unknown |
Society and culture
Legal status
and
The legal status of 5-MeO-MiPT differs internationally. It is not scheduled in Canada but is illegal if is sold for human consumption o recreational use ,[20] and in Luxembourg it is not listed among prohibited substances, making it not illegal but a legal gray area there.[21] In the United States, it is unscheduled at the federal level,[22] but may be treated as an analog of 5-MeO-DiPT under the Federal Analog Act therefore if is sold for human consumption and not for medical or scientific uses is illegal and persecuted. At the state level, “5-Methoxy-N-methyl-N-isopropyltryptamine” is classified as a Schedule I controlled substance in Florida, prohibiting its purchase, consumption, sale, or possession.[23]
In other jurisdictions, control is stricter and could be punished by death or life imprisonment its sale for human use. As of September–October 2015, China lists 5-MeO-MiPT as a controlled substance.[24] Finland includes it in its decree banning certain psychoactive substances from the consumer market.[25] In the United Kingdom, it is classified as a Class A drug along with most ethers of ring-hydroxy tryptamines.
Research
5-MeO-MiPT, under the developmental designation MSD-001, is being investigated for potential medical use. As of September 2024, it has received regulatory approval to begin Phase I clinical trials in healthy individuals in both the United States and the European Union.[26][27][28][29] The compound is being developed by Mindstate Design Labs, which uses an AI platform named Osmanthus to analyze subjective experience reports and identify relationships between receptor targets and psychoactive effects. MSD-001 is being developed as a neutral psychoactive base intended for combination with other compounds selected based on biochemical insights derived from this analysis.[30][31][32][33]
See also
- Substituted tryptamine
- 5-MeO-DiPT
- ASR-3001 (5-MeO-iPALT)
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 "#40 5-MEO-MIPT". Erowid Online Books: "TIHKAL". Retrieved 2021-06-01.
- ↑ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Aragón M (9 January 2024). "Meet Moxy: The Novel Psychedelic the DEA Tried To Ban". DoubleBlind Mag. Retrieved 8 October 2025.
- 1 2 3 4 Malaca S, Lo Faro AF, Tamborra A, Pichini S, Busardò FP, Huestis MA (December 2020). "Toxicology and Analysis of Psychoactive Tryptamines". Int J Mol Sci. 21 (23): 9279. doi:10.3390/ijms21239279. PMC 7730282. PMID 33291798.
5-Methoxy-N-methyl-N-isopropyltryptamine (5-MeO-MiPT): 5-MeO-MiPT or "moxy" was marketed as a "plant fertilizer." Oral doses ranged from 1–3 mg (light), 3–8 mg (common) and 8–12 mg (strong), with typical 10–20 mg doses if inhaled [22,103]. The principal effects lasted 3–7 h and included a general heightening of awareness, mild euphoria, psychedelic visual effects, such as enhanced colors but also anxiety, nausea, confusion and paranoia. Repke et al. studied if the effects of the drug would differ depending upon the route of administration [104]. If ingested the effects were stimulating, with visual hallucinations prevailing. 5-MeO-MiPT metabolism studied by LC-HRMS/MS identified six phase I metabolites following N-demethylation, O-demethylation, demethylation and hydroxylation and N-oxide formation and hydroxylation of the parent compound and N-O-bis-demethylation of the metabolite 5-OH-MiPT [105].
- 1 2 3 4 5 6 7 Palamar JJ, Acosta P (January 2020). "A qualitative descriptive analysis of effects of psychedelic phenethylamines and tryptamines". Human Psychopharmacology. 35 (1) e2719. doi:10.1002/hup.2719. PMC 6995261. PMID 31909513.
5-Meo-DIPT, Foxy, or Moxy (5-Meo-MIPT) are tryptamines that are distinct. A lot of people I know that like them think they're very aphrodisiac and much more stimulant and a party drug. My opinion on 5-MeO-MIPT is that it was a very sexual chemical … it feels good to touch things, and that sort of sensation certainly lends itself to sex but I wouldn't call it innately an aphrodisiac.
- 1 2 3 4 Rickli A, Moning OD, Hoener MC, Liechti ME (August 2016). "Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens" (PDF). European Neuropsychopharmacology. 26 (8): 1327–1337. doi:10.1016/j.euroneuro.2016.05.001. PMID 27216487. S2CID 6685927.
- 1 2 Puigseslloses P, Nadal-Gratacós N, Ketsela G, Weiss N, Berzosa X, Estrada-Tejedor R, et al. (August 2024). "Structure-activity relationships of serotonergic 5-MeO-DMT derivatives: insights into psychoactive and thermoregulatory properties". Molecular Psychiatry. 29 (8): 2346–2358. doi:10.1038/s41380-024-02506-8. PMC 11412900. PMID 38486047.
- 1 2 3 Ray TS (February 2010). "Psychedelics and the human receptorome". PLOS ONE. 5 (2) e9019. Bibcode:2010PLoSO...5.9019R. doi:10.1371/journal.pone.0009019. PMC 2814854. PMID 20126400.
- 1 2 Repke DB, Grotjahn DB, Shulgin AT (July 1985). "Psychotomimetic N-methyl-N-isopropyltryptamines. Effects of variation of aromatic oxygen substituents". Journal of Medicinal Chemistry. 28 (7): 892–896. doi:10.1021/jm00145a007. PMID 4009612.
- ↑ Luethi D, Liechti ME (October 2018). "Monoamine Transporter and Receptor Interaction Profiles in Vitro Predict Reported Human Doses of Novel Psychoactive Stimulants and Psychedelics". The International Journal of Neuropsychopharmacology. 21 (10): 926–931. doi:10.1093/ijnp/pyy047. PMC 6165951. PMID 29850881.
- ↑ Carpenter DE (2022-01-25). "DEA Proposes Adding Five Psychedelic Compounds to Schedule 1". Lucid News. Retrieved 2022-01-26.
- ↑ Baggott M (23 June 2023). Beyond Ecstasy: Progress in Developing and Understanding a Novel Class of Therapeutic Medicine. PS2023 [Psychedelic Science 2023, June 19–23, 2023, Denver, Colorado]. Denver, CO: Multidisciplinary Association for Psychedelic Studies.
- ↑ Hamilton Morris (28 November 2023). "POD 92: Understanding and Improving MDMA with Dr. Matthew Baggott". The Hamilton Morris Podcast (Podcast). Patreon. Retrieved 30 November 2024.
- ↑ "Borax Combo (Synonyms: Blue Bliss)". naddi.org. National Association of Drug Diversion Investigators (NADDI). 14 December 2022. Retrieved 21 November 2024.
- ↑ Altuncı YA, Aydoğdu M, Açıkgöz E, Düzağaç F, Atasoy A, Dağlıoğlu N, et al. (2021). "New Psychoactive Substance 5-MeO-MiPT in vivo Acute Toxicity and Hystotoxicological Study". Balkan Medical Journal. 38 (1): 34–42. doi:10.4274/balkanmedj.galenos.2020.2019.11.68. PMC 8909217. PMID 32936075.
- 1 2 3 Nagai F, Nonaka R, Satoh Hisashi Kamimura K (March 2007). "The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain". European Journal of Pharmacology. 559 (2–3): 132–137. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101.
- ↑ Blough BE, Landavazo A, Decker AM, Partilla JS, Baumann MH, Rothman RB (October 2014). "Interaction of psychoactive tryptamines with biogenic amine transporters and serotonin receptor subtypes". Psychopharmacology. 231 (21): 4135–4144. doi:10.1007/s00213-014-3557-7. PMC 4194234. PMID 24800892.
- ↑ Kozell LB, Eshleman AJ, Swanson TL, Bloom SH, Wolfrum KM, Schmachtenberg JL, et al. (April 2023). "Pharmacologic Activity of Substituted Tryptamines at 5-Hydroxytryptamine (5-HT)2A Receptor (5-HT2AR), 5-HT2CR, 5-HT1AR, and Serotonin Transporter". The Journal of Pharmacology and Experimental Therapeutics. 385 (1): 62–75. doi:10.1124/jpet.122.001454. PMC 10029822. PMID 36669875.
- ↑ Spratley T (2004). "Analytical Profiles for Five "Designer" Tryptamines" (PDF). Microgram Journal. 3 (1–2): 55. Retrieved 2013-10-09.
- ↑ "Controlled Drugs and Substances Act". Justice Laws Website. Government of Canada. Retrieved March 28, 2025.
- ↑ "Loi du 19 février 1973 concernant la vente de substances médicamenteuses et la lutte contre la toxicomanie" [Law of February 19, 1973 concerning the sale of medicinal substances and the fight against drug addiction]. Journal officiel du Grand-Duché de Luxembourg [Official Journal of the Grand Duchy of Luxembourg] (in French).
- ↑ "21 CFR — SCHEDULES OF CONTROLLED SUBSTANCES §1308.11 Schedule I." Archived from the original on 2009-08-27. Retrieved 2014-12-17.
- ↑ "Chapter 893 - Drug Abuse Prevention and Control". Florida Statutes.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" [Notice on Printing and Distributing the "Measures for the Scheduling of Non-Pharmaceutical Narcotic Drugs and Psychotropic Substances"] (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 1 October 2015.
- ↑ "FINLEX ® - Säädökset alkuperäisinä: Valtioneuvoston asetus kuluttajamarkkinoilta… 1130/2014". www.finlex.fi. Retrieved 11 July 2023.
- ↑ Ovalle D, Beard M (5 September 2024). "FDA gives an early nod to psychedelic research". The Washington Post. Retrieved 7 August 2025.
- ↑ NewsDesk M (10 September 2024). "Mindstate Design Labs AI-Designed Trial Gets FDA Approval". Microdose. Retrieved 10 November 2024.
- ↑ Ducharme J (2 October 2024). "Safer Psychedelic Drugs May Be Coming". TIME. Retrieved 7 August 2025.
- ↑ Bayer M (13 March 2024). "After crunching 70k 'trip reports', Mindstate looks to test first AI-derived psychedelic on humans". Fierce Biotech. Retrieved 10 November 2024.
- ↑ Meissen A (20 September 2024). "Mindstate Uses AI to Design "Next-Gen" Psychedelics Combined With 5-MeO-MiPT". Lucid News. Retrieved 10 November 2024.
- ↑ Nichols MR (20 June 2025). "The next era of psychedelics may be precision-designed". Big Think. Retrieved 7 August 2025.
- ↑ Dimitropoulos S (12 June 2025). "Science Has a Powerful New Tool to Unlock the Mysteries of Consciousness—And Even Help You Reach Transcendence". Popular Mechanics. Retrieved 7 August 2025.
- ↑ Houser K (28 May 2025). "Startups Are Trying to Hack Psychedelic Drugs to Make Them Safer". Futurism. Retrieved 7 August 2025.