5-MeO-MPMI
 | |
| Clinical data | |
|---|---|
| Other names | 5-Methoxy-N-methyl-(α,N-trimethylene)tryptamine; CP-108509; CP108509 |
| Drug class | Serotonergic psychedelic; Hallucinogen |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H20N2O |
| Molar mass | 244.338 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
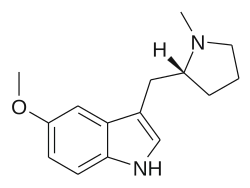
5-MeO-MPMI (developmental code name CP-108509), also known as 5-methoxy-N-methyl-(α,N-trimethylene)tryptamine, is a psychedelic drug of the pyrrolidinylmethylindole and cyclized tryptamine families.[1]
Interactions
Pharmacology
Pharmacodynamics
5-MeO-MPMI produces psychedelic-appropriate responding in animal tests, with similar potency to DOI.[1] It has two enantiomers, with only the (R)-enantiomer being active.[1]
History
5-MeO-MPMI was first developed by the team led by J. E. Macor and colleagues in 1992.[2] It was subsequently investigated by the team led by David E. Nichols from Purdue University in the late 1990s.[1]
See also
References
- 1 2 3 4 Gerasimov M, Marona-Lewicka D, Kurrasch-Orbaugh DM, Qandil AM, Nichols DE (1999). "Further studies on oxygenated tryptamines with LSD-like activity incorporating a chiral pyrrolidine moiety into the side chain". Journal of Medicinal Chemistry. 42 (20): 4257–4263. CiteSeerX 10.1.1.690.4941. doi:10.1021/jm990325u. PMID 10514296.
- ↑ Macor JE, Blake J, Fox CB, Johnson C, Koe BK, Lebel LA, Morrone JM, Ryan K, Schmidt AW, Schulz DW, et al. (1992). "Synthesis and serotonergic pharmacology of the enantiomers of 3-[(N-methylpyrrolidin-2-yl)methyl]-5-methoxy-1H-indole: discovery of stereogenic differentiation in the aminoethyl side chain of the neurotransmitter serotonin". Journal of Medicinal Chemistry. 35 (23): 4503–4505. doi:10.1021/jm00101a032. PMID 1447752.
External links
| Tryptamines |
|
|---|---|
| 4-Hydroxytryptamines and esters/ethers |
|
| 5-Hydroxy- and 5-methoxytryptamines |
|
| N-Acetyltryptamines |
|
| α-Alkyltryptamines |
|
| Cyclized tryptamines |
|
| Isotryptamines | |
| Related compounds |
|
| |
This article is issued from WikiProjectMed. The text is available under Creative Commons Attribution-ShareAlike License unless otherwise noted. Additional terms may apply for the media files.