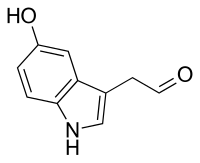
5-Hydroxyindoleacetaldehyde
 | |
| Names | |
|---|---|
| IUPAC name
2-(5-hydroxy-1H-indol-3-yl)acetaldehyde | |
| Other names
5-Hydroxyindole-acetaldehyde; 5-HIAL; 5-HIAAL; 5-Hydroxytryptaldehyde; 5-Hydroxyindole-3-acetaldehyde; Serotonin aldehyde[1] | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C10H9NO2 |
| Molar mass | 175.18 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
5-Hydroxyindoleacetaldehyde (5-HIAL), also known as 5-hydroxytryptaldehyde or as serotonin aldehyde, is an inactive metabolite and metabolic intermediate of the monoamine neurotransmitter serotonin (5-hydroxytryptamine; 5-HT).[2][3][1]
5-HIAL is formed from serotonin by oxidative deamination via monoamine oxidase (MAO).[2][3] MAO-mediated deamination is the primary metabolic pathway of serotonin inactivation.[2] Monoamine oxidase A (MAO-A) has about 120-fold higher affinity for serotonin than monoamine oxidase B (MAO-B).[2] In relation to this, MAO-A is the main isozyme of MAO involved in serotonin degradation.[2]
Following its formation, 5-HIAL is metabolized by aldehyde dehydrogenase (ALDH) to form 5-hydroxyindoleacetic acid (5-HIAA).[2][3] 5-HIAL can also be converted into small amounts of 5-hydroxytryptophol (5-HTOL; also known as 5-hydroxyindolethanol or 5-HIET) by either aldehyde reductase (ALR/ALDR) or alcohol dehydrogenase (ADH).[2][4] However, brain concentrations of 5-HTOL are only 1 to 5% of those of 5-HIAA.[2][4]
Use of ethanol (alcohol) can dramatically increase 5-HTOL formation by inhibiting ALDH and enhancing ADH activity.[2][5] As a result, the ratio of 5-HTOL to 5-HIAA is a sensitive and reliable marker of recent ethanol ingestion and has been suggested for use in clinical and forensic contexts.[2][5]
Besides oxidative deamination by MAO into 5-HIAL, serotonin can also be conjugated by glucuronidation via glucuronyltransferases, conjugated by sulfation via sulfotransferases, acetylated and then methylated into melatonin (N-acetyl-5-methoxytryptamine) (which occurs mainly in the pineal gland), and converted into certain other metabolites like 5-hydroxyindole thiazoladine carboxylic acid (5-HITCA).[2] However, these secondary metabolic pathways appear to play only a minor role in serotonin metabolism.[2]
5-HIAL has been implicated in producing neurotoxicity and in the development and progression of neurodegenerative diseases.[6][7][8]
See also
- Indoleacetaldehyde (IAL)
- 3,4-Dihydroxyphenylacetaldehyde (DOPAL)
- 3,4-Dihydroxyphenylglycolaldehyde (DOPEGAL)
References
- 1 2 Jinsmaa Y, Cooney A, Sullivan P, Sharabi Y, Goldstein DS (March 2015). "The serotonin aldehyde, 5-HIAL, oligomerizes alpha-synuclein". Neurosci Lett. 590: 134–137. doi:10.1016/j.neulet.2015.01.064. PMC 4755587. PMID 25637699.
- 1 2 3 4 5 6 7 8 9 10 11 12 Bortolato, Marco; Chen, Kevin; Shih, Jean C. (2010). "The Degradation of Serotonin: Role of MAO". Handbook of Behavioral Neuroscience. Vol. 21. Elsevier. pp. 203–218. doi:10.1016/s1569-7339(10)70079-5. ISBN 978-0-12-374634-4.
- 1 2 3 Matthes S, Mosienko V, Bashammakh S, Alenina N, Bader M (2010). "Tryptophan hydroxylase as novel target for the treatment of depressive disorders". Pharmacology. 85 (2): 95–109. doi:10.1159/000279322. PMID 20130443.
- 1 2 Bortolato M, Shih JC (2011). "Behavioral outcomes of monoamine oxidase deficiency: preclinical and clinical evidence". Int Rev Neurobiol. International Review of Neurobiology. 100: 13–42. doi:10.1016/B978-0-12-386467-3.00002-9. ISBN 978-0-12-386467-3. PMC 3371272. PMID 21971001.
- 1 2 Beck O, Helander A (December 2003). "5-hydroxytryptophol as a marker for recent alcohol intake". Addiction. 98 Suppl 2: 63–72. doi:10.1046/j.1359-6357.2003.00583.x. PMID 14984243.
- ↑ Cagle BS, Crawford RA, Doorn JA (February 2019). "Biogenic Aldehyde-Mediated Mechanisms of Toxicity in Neurodegenerative Disease". Curr Opin Toxicol. 13: 16–21. Bibcode:2019COTox..13...16C. doi:10.1016/j.cotox.2018.12.002. PMC 6625780. PMID 31304429.
- ↑ Matveychuk D, MacKenzie EM, Kumpula D, Song MS, Holt A, Kar S, Todd KG, Wood PL, Baker GB (January 2022). "Overview of the Neuroprotective Effects of the MAO-Inhibiting Antidepressant Phenelzine". Cell Mol Neurobiol. 42 (1): 225–242. doi:10.1007/s10571-021-01078-3. PMC 8732914. PMID 33839994.
- ↑ Behl T, Kaur D, Sehgal A, Singh S, Sharma N, Zengin G, Andronie-Cioara FL, Toma MM, Bungau S, Bumbu AG (June 2021). "Role of Monoamine Oxidase Activity in Alzheimer's Disease: An Insight into the Therapeutic Potential of Inhibitors". Molecules. 26 (12): 3724. doi:10.3390/molecules26123724. PMC 8234097. PMID 34207264.