Isoproscaline
 | |
| Names | |
|---|---|
| Preferred IUPAC name
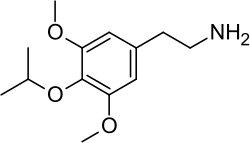
2-{{#parsoidfragment:0}}{3,5-Dimethoxy-4-[(propan-2-yl)oxy]phenyl}ethan-1-amine | |
| Other names
2-(4-Isopropoxy-3,5-dimethoxyphenyl)ethanamine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C13H21NO3 |
| Molar mass | 239.31 g/mol |
| Melting point | 163 to 164 °C (325 to 327 °F; 436 to 437 K) (hydrochloride) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Isoproscaline or 4-isopropoxy-3,5-dimethoxyphenethylamine is an analog of mescaline. It is closely related to proscaline and was first synthesized by David E. Nichols and colleagues.[1] It produces hallucinogenic, psychedelic, and entheogenic effects.
Use and effects
Little is known about the psychopharmacological effects of isoproscaline. In his book PiHKAL, Alexander Shulgin lists a psychedelic dose as being 40–80 mg, with effects lasting 10–16 hours.[2]
Interactions
Pharmacology
Pharmacodynamics
The mechanism that produces the hallucinogenic and entheogenic effects of isoproscaline is most likely to result from action as a 5-HT2A serotonin receptor agonist in the brain, a mechanism of action shared by all of the hallucinogenic tryptamines and phenethylamines.
Chemistry
Isoproscaline is in a class of compounds commonly known as phenethylamines, and the full chemical name is 2-(4-isopropoxy-3,5-dimethoxyphenyl)ethanamine.
Society and culture
Legal status
Isoproscaline is unscheduled in the United States; however, because of its close similarity in structure and effects to mescaline, possession and sale of isoproscaline may be subject to prosecution under the Federal Analog Act.
In the UK, its highly likely that this compound would be covered by the "phenylethylamine amendment" to the misuse of drugs act likely rendering it a Class A controlled drug.
See also
References
- ↑ Nichols DE, Dyer DC (February 1977). "Lipophilicity and serotonin agonist activity in a series of 4-substituted mescaline analogues". J Med Chem. 20 (2): 299–301. doi:10.1021/jm00212a022. PMID 836502.
- ↑ Isoproscaline entry in PiHKAL
| Phenethylamines |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphetamines |
| ||||||||||||||||
| Phentermines |
| ||||||||||||||||
| Cathinones |
| ||||||||||||||||
| Phenylisobutylamines (and further-extended) | |||||||||||||||||
| Catecholamines (and close relatives) |
| ||||||||||||||||
| Cyclized phenethylamines |
| ||||||||||||||||
| Related compounds |
| ||||||||||||||||
| |||||||||||||||||