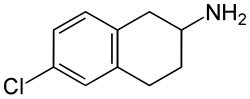
6-CAT
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
6-Chloro-2-aminotetralin (6-CAT) is a drug which acts as a selective serotonin releasing agent (SSRA) and is a putative entactogen in humans.[1][2] It is a rigid analogue of para-chloroamphetamine (PCA).[1]
According to Nichols et al.,[3] 6-CAT is a non-neurotoxic analog of PCA.
Other related compounds that are creditworthy of mention include 6,7-DCAT[4] & 5,6-DCAT [57915-89-6].[4] These compounds were invented by Bryan Molloy (the inventor of Prozac). These can be thought to be rigid analogs of 3,4-Dichloroamphetamine and might be predicted to be similarly non-neurotoxic. A positional isomer of the aforementioned two compounds is also known to exist in the literature with a 5,8-dichloro substitution pattern (PC9954388).[5] The compound depicted in the latter case is with the spirodecanone pharmacophore.
Synthesis
Although more modern discussions on chemical synthesis are possible, Bryan Molloy lays the groundwork to a preliminary drug discovery phase of development.
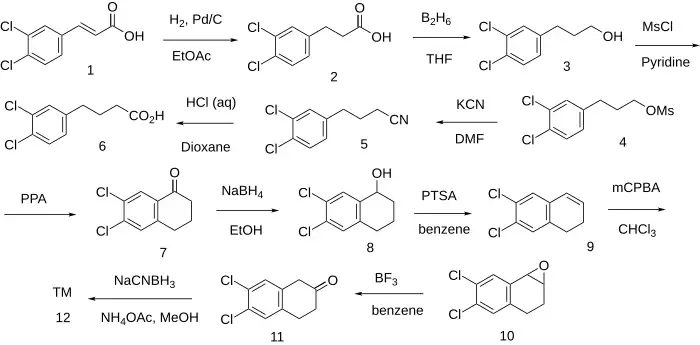
The catalytic hydrogenation of 3,4-Dichlorocinnamic acid [1202-39-7] [7312-27-8] (1) gave 3-(3,4-Dichlorophenyl)Propanoic acid [25173-68-6] (2). The reduction of the acid with diborane led to 3-(3,4-Dichlorophenyl)propanol [39960-05-9] (3). Treatment with mesyl chloride gave 3-(3,4-dichlorophenyl)propyl methanesulfonate, PC131855432 (4). FGI to the nitrile gave 4-(3,4-Dichlorophenyl)Butanenitrile [39960-06-0] (5). This was then hydrolyzed in aqueous base giving 4-(3,4-Dichlorophenyl)Butanoic Acid [25157-66-8] (6). Cyclization was effected in the presence of PPA giving 6,7-Dichloro-1-tetralone [25095-57-2] (7). Sodium borohydride reduction of the ketone into an alcohol gave 6,7-dichloro-1,2,3,4-tetrahydronaphthalen-1-ol, PC82027291 (8). Dehydration of the alcohol to an olefin by refluxing in tosic acid provided (9). Treatment with peroxy acid formed the oxirane (10). Rearrangement of the epoxide occurred upon treatment with boron trifluoride to give 6,7-Dichloro-2-Tetralone [17556-22-8] (11). Reductive amination completed the practical (12).
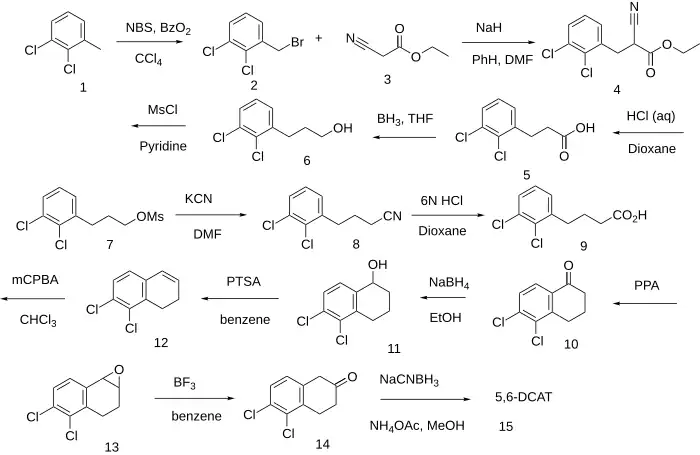
The Free-radical halogenation of 1,2-Dichloro-3-methylbenzene [32768-54-0] (1) gives 2,3-Dichlorobenzyl Bromide [57915-78-3] (2). Base induced alkylation with ethyl cyanoacetate [105-56-6] (3) gives ethyl 3-(2,3-dichlorophenyl)-2-cyanopropionate [39959-98-3] (4). Acid induced saponification of the ester, hydrolysis of the nitrile and subsequent decarboxylation arrives at 3-(2,3-dichlorophenyl)propionic acid [57915-79-4] (5). Diborane reduction of the acid gives 3-(2,3-dichlorophenyl)propanol [57915-80-7] (6). FGI to the mesylate gives (7). Displacement of the leaving group by cyanide gives 4-(2,3-dichlorophenyl)butanenitrile, PC53434490 (8). Acid hydrolysis of the nitrile to the acid gives 4-(2,3-dichlorophenyl)butanoic acid, PC14976589 (9). PPA intramolecular cyclization gives 5,6-Dichloro-1-tetralone [57915-84-1] (10). Sodium borohydride reduction of the keto group gives 5,6-Dichloro-1-tetralol (11). The dehydration of the alcohol occurs in the presence of acid to leave 5,6-Dichloro-1-tetralene (12). Peroxyacid oxidation of the olefin to the oxirane gives 5,6-Dichloro-1,2-epoxy-tetralin, PC154248247 (13). Rearrangement of the epoxide to the 2-tetralone in the presence of boron trifluoride gives 5,6-Dichloro-2-tetralone [57915-88-5] (14). Reductive amination with ammonium acetate and sodium cyanoborohydride completed the synthesis of the title compound (15).
Also notice a related agent called SKF-64139 & LY-134046.
See also
- 2-Aminotetralin
References
- 1 2 Fuller RW, Perry KW, Baker JC, Molloy BB (November 1974). "6-Chloro-2-aminotetralin, a rigid Conformational analog of 4-chloroamphetamine: pharmacologic properties of it and related compounds in rats". Archives Internationales de Pharmacodynamie et de Therapie. 212 (1): 141–53. PMID 4455127.
- ↑ Fuller RW, Wong DT, Snoddy HD, Bymaster FP (1977). "Comparison of the effects of 6-chloro-2-aminotetralin and of ORG 6582, a related chloroamphetamine analog, on brain serotonin metabolism in rats". Biochemical Pharmacology. 26 (1): 1, 333–1, 337. doi:10.1016/0006-2952(77)90094-6.
- ↑ Johnson MP, Frescas SP, Oberlender R, Nichols DE (May 1991). "Synthesis and pharmacological examination of 1-(3-methoxy-4-methylphenyl)-2-aminopropane and 5-methoxy-6-methyl-2-aminoindan: similarities to 3,4-(methylenedioxy)methamphetamine (MDMA)". Journal of Medicinal Chemistry. 34 (5): 1662–8. doi:10.1021/jm00109a020. PMID 1674539.
- 1 2 Bryan B Molloy, U.S. patent 3,919,316 (1975 to Eli Lilly and Co).
- ↑ Röver, S., Adam, G., Cesura, A. M., Galley, G., Jenck, F., Monsma, F. J., Wichmann, J., Dautzenberg, F. M. (6 April 2000). "High-Affinity, Non-Peptide Agonists for the ORL1 (Orphanin FQ/Nociceptin) Receptor". Journal of Medicinal Chemistry. 43 (7): 1329–1338. doi:10.1021/jm991129q.