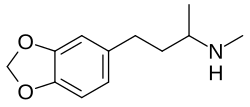
Homo-MDMA
 | |
| Clinical data | |
|---|---|
| Other names | HMDMA; MDP-3-MB; α,N-Dimethyl-3-(3,4-methylenedioxyphenyl)propylamine; α,N-Dimethyl-1,3-benzodioxole-5-propylamine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H17NO2 |
| Molar mass | 207.273 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Homo-MDMA (HMDMA), also known as α,N-dimethyl-3-(3,4-methylenedioxyphenyl)propylamine, is an entactogen-like drug of the phenylpropylamine group related to MDMA.[1][2] It is an analogue of MDMA in which the side chain has been lengthened by one carbon atom.[1][2]
It showed very weak induction of serotonin release (much less than that of MDMA or methamphetamine) and no significant release of dopamine in rat brain synaptosomes.[1][3] As such, its monoamine-releasing activity was said to have been essentially abolished relative to MDMA.[3] The drug partially substituted for MDMA in rodent drug discrimination tests but produced seizures at high doses.[1][3]
Based on unpublished findings by Alexander Shulgin, homo-MDMA has been said to be inactive in humans.[1][3] However, it has been encountered as a designer and recreational drug in Japan and was being sold as "MBDB".[1][4] It is not a controlled substance in the United States as of 2011.[1]
See also
References
- 1 2 3 4 5 6 7 Shulgin A, Manning T, Daley PF (2011). "#83. Homo-MDMA". The Shulgin Index, Volume One: Psychedelic Phenethylamines and Related Compounds. Vol. 1. Berkeley, CA: Transform Press. pp. 201–202. ISBN 978-0-9630096-3-0. OCLC 709667010.
- 1 2 Bronson ME, Barrios-Zambrano L, Jiang W, Clark CR, DeRuiter J, Newland MC (December 1994). "Behavioral and developmental effects of two 3,4-methylenedioxymethamphetamine (MDMA) derivatives". Drug Alcohol Depend. 36 (3): 161–166. doi:10.1016/0376-8716(94)90141-4. PMID 7889806.
- 1 2 3 4 McKenna DJ, Guan XM, Shulgin AT (March 1991). "3,4-Methylenedioxyamphetamine (MDA) analogues exhibit differential effects on synaptosomal release of 3H-dopamine and 3H-5-hydroxytryptamine". Pharmacol Biochem Behav. 38 (3): 505–512. doi:10.1016/0091-3057(91)90005-m. PMID 1829838.
Extension of the side-chain of MDMA by one carbon to give the homologue HMDMA (41), or replacement of one methylenedioxy oxygen with sulfur to give the compound 4-T-MMDA-2 (Fig. 1) essentially abolishes the 3H-5-HT and 3H-DA releasing capability, as well as the central psychotropic effect in man (Shulgin and Jacob, unpublished). The possible neurotoxicity of these analogues is not addressed in the present study.
- ↑ Matsumoto T, Kikura-Hanajiri R, Kamakura H, Kawahara N, Goda Y (2006). "Identification of N-Methyl-4-(3,4-Methylenedioxyphenyl)Butan-2-Amine, Distributed as MBDB". Journal of Health Science. 52 (6): 805–810. doi:10.1248/jhs.52.805. ISSN 1344-9702. Retrieved 3 February 2025.
External links