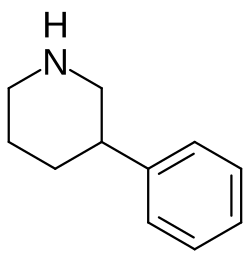
3-Phenylpiperidine
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.021.458 |
| Chemical and physical data | |
| Formula | C11H15N |
| Molar mass | 161.248 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
3-Phenylpiperidine is a chemical compound and cyclized phenethylamine.[1] It can be thought of as β-phenethylamine with a propyl group connecting the amine and the β position.[1]
3-Phenylpiperidine is a parent compound of several drugs such as the psychedelic and related drugs LPH-5[2] and Z3517967757 (Z7757),[3] the antipsychotic OSU-6162,[4] and the sigma receptor agonist 3-PPP.[1]
According to Daniel Trachsel and colleagues in 2013, the pharmacology of 3-phenylpiperidine itself has not been reported, only its chemical synthesis has been described.[1][5] 3-Phenylpiperidine was first described in the scientific literature by 1933.[1][5]
See also
- Phenylpiperidines
- 4-Phenylpiperidine
- 2-Methyl-3-phenylpiperidine
- Phenmetrazine
- Substituted phenylmorpholine
- 1-Phenylpiperazine
- Partial ergoline
References
- 1 2 3 4 5 Trachsel D, Lehmann D, Enzensperger C (2013). Phenethylamine: von der Struktur zur Funktion [Phenethylamines: From Structure to Function]. Nachtschatten-Science (in German) (1 ed.). Solothurn: Nachtschatten-Verlag. ISBN 978-3-03788-700-4. OCLC 858805226.
Das Piperidinring-Positionsisomer 3-Phenylpiperidin (68) enthält die Phenethylamin-Substruktur. Es wurde bis anhin nur synthetisch beschrieben [56]. Das Derivat 69 ist unter dem Namen 3-PPP bekannt. Es ist für seine Affinität zum Sigma-Rezeptor bekannt und wurde Z.B. daraufhin untersucht, inwieweit es die antikonvulsive Wirkung von herkömmlichen Antiepileptika beeinflusst [97]. Zudem bindet es an Dopamin D2-Rezeptoren [98]. Das (+)-Enantiomer wirkt schwach agonistisch und das (-)-Enantiomer antagonistisch [98].
- ↑ M Ro Rsted E, Jensen AA, Smits G, Frydenvang K, Kristensen JL (May 2024). "Discovery and Structure-Activity Relationships of 2,5-Dimethoxyphenylpiperidines as Selective Serotonin 5-HT2A Receptor Agonists". Journal of Medicinal Chemistry. 67 (9): 7224–7244. doi:10.1021/acs.jmedchem.4c00082. PMC 11089506. PMID 38648420.
- ↑ Lyu J, Kapolka N, Gumpper R, Alon A, Wang L, Jain MK, et al. (June 2024). "AlphaFold2 structures guide prospective ligand discovery". Science. 384 (6702) eadn6354. New York, N.Y. Bibcode:2024Sci...384n6354L. doi:10.1126/science.adn6354. PMC 11253030. PMID 38753765.
- ↑ Natesan S, Svensson KA, Reckless GE, Nobrega JN, Barlow KB, Johansson AM, et al. (August 2006). "The dopamine stabilizers (S)-(-)-(3-methanesulfonyl-phenyl)-1-propyl-piperidine [(-)-OSU6162] and 4-(3-methanesulfonylphenyl)-1-propyl-piperidine (ACR16) show high in vivo D2 receptor occupancy, antipsychotic-like efficacy, and low potential for motor side effects in the rat". The Journal of Pharmacology and Experimental Therapeutics. 318 (2): 810–818. doi:10.1124/jpet.106.102905. PMID 16648369.
- 1 2 Walters LA, McElvain SM (1933). "Piperidine Derivatives. XIII. Phenyl and Phenylalkyl Substituted Piperidinopropyl Benzoates". Journal of the American Chemical Society. 55 (11): 4625–4629. Bibcode:1933JAChS..55.4625W. doi:10.1021/ja01338a049. ISSN 0002-7863. Retrieved 13 July 2025.
External links