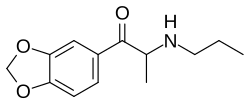
Propylone
 | |
| Clinical data | |
|---|---|
| Other names | 3,4-Methylenedioxy-N-propylcathinone; MD-PrC; PrONE; bk-3,4-MDPA |
| Drug class | Monoamine releasing agent; Monoamine reuptake inhibitor |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C13H17NO3 |
| Molar mass | 235.283 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Propylone, also known as 3,4-methylenedioxy-N-propylcathinone is a mixed monoamine releasing agent and reupake inhibitor of the cathinone family related to methylone and ethylone.[1]
It acts specifically as a weak partial serotonin–dopamine releasing agent (SDRA) and serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), with EC50 (Emax) values for induction of monoamine release of 3,128 nM (30%) for serotonin and 976 nM (20%) for dopamine, and IC50 value for monoamine reuptake inhibition of 2,462 nM for serotonin, 28,540 nM for norepinephrine, and 1,863 nM for dopamine.[1]
The drug was first described in the literature in a 1996 patent by Alexander Shulgin and Peyton Jacob III.[2] It was subsequently characterized more thoroughly by 2015[1] and was encountered as a novel designer drug in Europe by 2016.[3][4][5]
See also
References
- 1 2 3 Sakloth F (11 December 2015). Psychoactive synthetic cathinones (or 'bath salts'): Investigation of mechanisms of action. Theses and Dissertations (Ph.D. thesis). Virginia Commonwealth University. doi:10.25772/AY8R-PW77. Retrieved 24 November 2024 – via VCU Scholars Compass.
- ↑ WO 1996039133, Jacob III P, Shulgin AT, "Preparation of novel N-substituted-2-amino-3′,4′-methylenedioxypropiophenones as anti-depressant and anti-parkinsonism agents.", published 12 December 1996, assigned to Neurobiological Technologies, Inc.
- ↑ "EMCDDA–Europol 2016 Annual Report on the implementation of Council Decision 2005/387/JHA". Europol. 23 October 2018. Retrieved 11 March 2025.
- ↑ Liu C, Jia W, Li T, Hua Z, Qian Z (August 2017). "Identification and analytical characterization of nine synthetic cathinone derivatives N-ethylhexedrone, 4-Cl-pentedrone, 4-Cl-α-EAPP, propylone, N-ethylnorpentylone, 6-MeO-bk-MDMA, α-PiHP, 4-Cl-α-PHP, and 4-F-α-PHP". Drug Test Anal. 9 (8): 1162–1171. doi:10.1002/dta.2136. PMID 27863142.
- ↑ Pulver B, Fischmann S, Gallegos A, Christie R (December 2024). "EMCDDA framework and practical guidance for naming cathinones". Drug Test Anal. 16 (12): 1409–1435. doi:10.1002/dta.3662. PMC 11635063. PMID 38389255.
External links