Zalsupindole
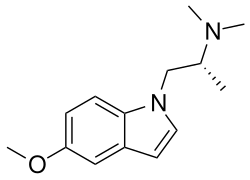 | |
 Above: structure of zalsupindole
Below: 3D representation of a zalsupindole molecule | |
| Clinical data | |
|---|---|
| Other names | ZAL; DLX-001; DLX-1; DLX001; DLX1; AAZ-A-154; AAZ; (R)-5-Methoxy-N,N-dimethyl-α-methylisotryptamine; (R)-5-MeO-α-methyl-isoDMT; (R)-5-MeO-N,N-dimethyl-isoAMT |
| Routes of administration | Oral[1][2][3] |
| Drug class | Non-hallucinogenic serotonin 5-HT2A receptor agonist; Psychoplastogen |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H20N2O |
| Molar mass | 232.327 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Zalsupindole, also known by its code names DLX-001 and AAZ-A-154 and as (R)-5-methoxy-N,N-dimethyl-α-methylisotryptamine ((R)-5-MeO-α-Me-isoDMT), is non-hallucinogenic serotonin receptor agonist and psychoplastogen of the isotryptamine family related to psychedelic tryptamines such as dimethyltryptamine (DMT).[1][2][4][5][6] It is under development for the treatment of major depressive disorder and other central nervous system disorders.[2][4] The drug is taken orally.[1][2][3]
It acts as a partial agonist of the serotonin 5-HT2A receptor and also interacts with other serotonin receptors.[1] The drug activates the serotonin 5-HT2A receptor with sufficiently high efficacy to promote neuroplasticity but not with adequate efficacy to cause psychedelic effects.[1] It does not produce psychedelic-like effects in animals or humans but does produce antidepressant-like effects in animals.[1][3]
Zalsupindole was first described in the scientific literature by 2021.[7] It was developed by David E. Olson and colleagues at the University of California, Davis and Delix Therapeutics.[2][4] As of December 2024, it is in phase 1 clinical trials and a phase 2 trial is being planned.[2]
Use and effects
A phase 1 dose-ranging clinical trial found that zalsupindole is non-hallucinogenic in humans across a dose range of 2 to 360 mg orally.[1][3] Nonetheless, it produced changes in brain function as measured by quantitative electroencephalography (qEEG).[3]
Side effects
Side effects of zalsupindole include dose-dependent nausea, headache, and dizziness.[3]
Pharmacology
Pharmacodynamics
Zalsupindole is a non-selective serotonin receptor modulator including of the serotonin 5-HT2A receptor.[1][8] It acts as a low-potency, low-efficacy partial agonist of the serotonin 5-HT2A receptor, with an EC50 of 8,200 nM and an Emax of 17%.[1] The drug is also a moderate-efficacy partial agonist of the serotonin 5-HT2C receptor, with an EC50 of 3,300 nM and an Emax of 70%.[1] Other activities have also been reported.[1] It is selective for the serotonin 5-HT2 receptors over a number of other receptors, including dopamine receptors, adrenergic receptors, and the κ-opioid receptor, among others.[1][8] The drug is a silent antagonist of the serotonin 5-HT2B receptor, with an IC50 of 27,600 nM.[1][6]
Zalsupindole is orally bioavailable and centrally penetrant in animals.[6] It is a psychoplastogen via activation of the serotonin 5-HT2A receptor and rapidly and persistently increases neuroplasticity in preclinical research.[1][9][5][6] Animal studies have found that it produces antidepressant-like effects without causing psychedelic-like effects such as the head-twitch response.[1][7][10][5][6][11] It does not produce hyperlocomotion at therapeutically relevant doses.[1] In accordance with its serotonin 5-HT2B receptor antagonism, zalsupindole showed no cardiovascular safety signals in animals.[6]
Pharmacokinetics
The pharmacokinetics of zalsupindole have been studied.[1][3]
Chemistry
Zalsupindole, also known as (R)-5-methoxy-N,N-dimethyl-α-methylisotryptamine, is a substituted isotryptamine derivative.[12][13] It is a combined derivative of 5-methoxy-N,N-dimethylisotryptamine (5-MeO-isoDMT) and α-methylisotryptamine (isoAMT).[12][13] Another related compound is 6-methoxy-N,N-dimethylisotryptamine (6-MeO-isoDMT).[12] Zalsupindole is a close isotryptamine analogue of α,N,N,O-tetramethylserotonin (α,N,N,O-TMS or 5-MeO-α,N,N-TMT).[13]
History
Zalsupindole was first described in the scientific literature by David E. Olson and colleagues in 2021.[7] It was developed by Olson's lab at the University of California, Davis and at his company Delix Therapeutics.[2][4] The drug was initially described under the name AAZ-A-154 and then by the name DLX-001 before receiving the name zalsupindole.[2][4][1]
Society and culture
Names
Zalsupindole is the generic name of the drug and its INN.[14] It is also known by its developmental code names DLX-001 and AAZ-A-154.[2][4]
Research
Zalsupindole, as well as related drugs such as tabernanthalog (TBG; DLX-007), DLX-159, DLX-2270, and JRT, are licensed by Delix Therapeutics and are being developed for treatment of neuropsychiatric disorders such as depression and schizophrenia.[9][2][4] As of December 2024, zalsupindole is in phase 1 clinical trials for major depressive disorder and other central nervous system disorders.[2][4] A phase 2 trial is being planned.[2]
See also
- Substituted isotryptamine
- Non-hallucinogenic 5-HT2A receptor agonist
- List of investigational hallucinogens and entactogens
- List of investigational antidepressants
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Agrawal R, Gillie D, Mungenast A, Chytil M, Engel S, Wu MC, et al. (October 2025). "Zalsupindole is a Nondissociative, Nonhallucinogenic Neuroplastogen with Therapeutic Effects Comparable to Ketamine and Psychedelics". ACS Chem Neurosci acschemneuro.5c00667. doi:10.1021/acschemneuro.5c00667. PMID 41078264.
- 1 2 3 4 5 6 7 8 9 10 11 12 "DLX 1". AdisInsight. 11 December 2023. Retrieved 2 November 2024.
- 1 2 3 4 5 6 7 Koenig A, van der Aa L, Pelletier N, Patat A, Viardot G, Olson D, et al. (2024). "ACNP 63rd Annual Meeting: Poster Abstracts P1-P304: P163. Phase I Results for DLX-001, a Novel Neuroplastogen Under Development for the Treatment of Major Depressive Disorder" (PDF). Neuropsychopharmacology. 49 (S1): 65–235 (157–158). doi:10.1038/s41386-024-02011-0. ISSN 0893-133X. PMC 11627186. PMID 39643633. Retrieved 31 January 2025.
- 1 2 3 4 5 6 7 8 "Delving into the Latest Updates on DLX-001 with Synapse". Synapse. 1 November 2024. Retrieved 2 November 2024.
- 1 2 3 Rasmussen K, Chytil M, Agrawal R, Leach P, Gillie D, Mungenast A, et al. (2024). "14. Preclinical Pharmacology of DLX-001, a Novel Non-Hallucinogenic Neuroplastogen With the Potential for Treating Neuropsychiatric Diseases". Biological Psychiatry. 95 (10). Elsevier BV: S80. doi:10.1016/j.biopsych.2024.02.192. ISSN 0006-3223.
- 1 2 3 4 5 6 Rasmussen K, Engel S, Chytil M, Koenig A, Meyer R, Rus M, et al. (December 2023). "ACNP 62nd Annual Meeting: Poster Abstracts P251 - P500: P361. Preclinical Pharmacology of DLX-001, a Novel Non-Hallucinogenic Neuroplastogen With the Potential for Treating Neuropsychiatric Diseases". Neuropsychopharmacology. 48 (Suppl 1): 211–354 (274–275). doi:10.1038/s41386-023-01756-4. PMC 10729596. PMID 38040810.
- 1 2 3 Dong C, Ly C, Dunlap LE, Vargas MV, Sun J, Hwang IW, et al. (May 2021). "Psychedelic-inspired drug discovery using an engineered biosensor". Cell. 184 (10): 2779–2792.e18. doi:10.1016/j.cell.2021.03.043. PMC 8122087. PMID 33915107.
- 1 2 Dunlap LE (2022). Development of Non-Hallucinogenic Psychoplastogens (Thesis). University of California, Davis. Retrieved 18 November 2024.
- 1 2 "Can we take the high out of psychedelics?". Wired UK. ISSN 1357-0978. Retrieved 2022-07-07.
- ↑ WO 2020176597, Olson DE, Dunlap L, Wagner FF, "N-substituted indoles and other heterocycles for treating brain disorders", published 3 September 2020, assigned to The Regents of the University of California
- ↑ Cross R (2021-09-27). "Delix raises $70 million to synthesize psychedelic-inspired drugs". cen.acs.org. Archived from the original on 2021-09-27. Retrieved 2022-01-14.
- 1 2 3 Duan W, Cao D, Wang S, Cheng J (January 2024). "Serotonin 2A Receptor (5-HT2AR) Agonists: Psychedelics and Non-Hallucinogenic Analogues as Emerging Antidepressants". Chem Rev. 124 (1): 124–163. doi:10.1021/acs.chemrev.3c00375. PMID 38033123.
- 1 2 3 "(2R)-1-(5-Methoxyindol-1-yl)-N,N-dimethylpropan-2-amine". PubChem. Retrieved 27 November 2024.
- ↑ https://iris.who.int/bitstream/handle/10665/380497/9789240107038-eng.pdf "zalsupindolum zalsupindole (2R)-1-(5-methoxy-1H-indol-1-yl)-N,N-dimethylpropan-2-amine antidepressant"