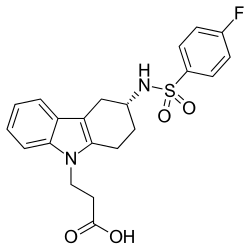
Ramatroban
 | |
| Clinical data | |
|---|---|
| Trade names | Baynas |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral (tablets) |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.159.668 |
| Chemical and physical data | |
| Formula | C21H21FN2O4S |
| Molar mass | 416.47 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ramatroban (INN; also known as BAY u3405)[1] is a thromboxane receptor antagonist.[2] It is also a DP2 receptor antagonist.[3]
Ramatroban is indicated for the treatment of coronary artery disease.[4] It has also been used for the treatment of asthma.[5]
It has been suggested that ramatroban, by modulating DP2 receptor, can reverse viremia-associated proinflammatory and prothrombotic processes which are similar to those induced by SARS-Cov-2. Hence, ramatroban, that has been used for the treatment of allergic rhinitis in Japan for the past two decades with a well established safety profile, merits investigation as a novel immunotherapy for the treatment of COVID-19 disease, although no clinical trial has yet been conducted.[6]
Ramatroban was developed by the German pharmaceutical company Bayer AG and is co-marketed in Japan by Bayer Yakuhin then marketed by Kyorin Pharmaceutical and Nippon Shinyaku Co., Ltd. under the trade name Baynas.
It is a tetrahydrocarbazolamine derivative and cyclized tryptamine.
Synthesis
The synthesis has been described:[7][8][9] Cmp#4[10] Patent:[11]
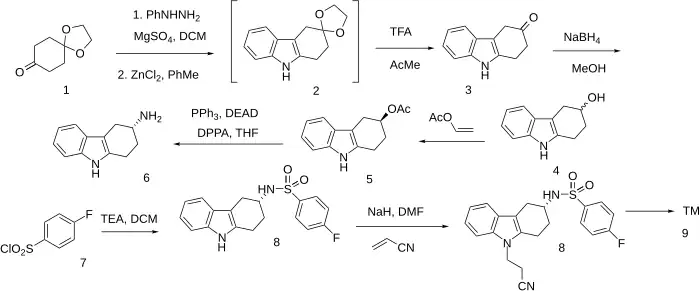
The starting material is called 1,4-cyclohexanedione monoethylene glycol ketal aka 1,4-Dioxaspiro[4.5]decan-8-one [4746-97-8]. The Borsche–Drechsel cyclization between (1) and Phenylhydrazine gives 5-Oxo-tetrahydrocarbazole ethylene ketal [54621-12-4] (2). Hydrolysis of the ketal protecting group gives 1,2,4,9-tetrahydrocarbazol-3-one [51145-61-0] (3). Reduction of the ketone with sodium borohydride gives 2,3,4,9-tetrahydro-1H-carbazol-3-ol [14384-34-0] (4). Acetylation by treatment with vinyl acetate [108-05-4] gives (3R)-3beta-Acetoxy-1,2,3,4-tetrahydro-9H-carbazole, PC59051734 (5a) & (3S)-1,2,3,4-Tetrahydro-9H-carbazole-3-ol, PC8142712 (5b). These can be separated at this stage into pure (S) for the next step. A Mitsunobu reaction in the presence of DPPA leads to an Azide with pure Walden inversion kinetics w/o racemization. The Staudinger reduction of the azide in situ gives (R)-3-Amino-1,2,3,4-tetrahydrocarbazole [874-937-6] [116650-33-0] (6).
References
- ↑ "Ramatroban (compound)". PubChem. National Center for Biotechnology Information. Retrieved 22 June 2019.
- ↑ Sugimoto H, Shichijo M, Iino T, Manabe Y, Watanabe A, Shimazaki M, et al. (April 2003). "An orally bioavailable small molecule antagonist of CRTH2, ramatroban (BAY u3405), inhibits prostaglandin D2-induced eosinophil migration in vitro". The Journal of Pharmacology and Experimental Therapeutics. 305 (1): 347–352. doi:10.1124/jpet.102.046748. PMID 12649388. S2CID 10016709.
- ↑ Royer JF, Schratl P, Carrillo JJ, Jupp R, Barker J, Weyman-Jones C, et al. (September 2008). "A novel antagonist of prostaglandin D2 blocks the locomotion of eosinophils and basophils". European Journal of Clinical Investigation. 38 (9): 663–671. doi:10.1111/j.1365-2362.2008.01989.x. PMID 18837743.
- ↑ Fiedler VB, Seuter F, Perzborn E (December 1990). "Effects of the novel thromboxane antagonist Bay U 3405 on experimental coronary artery disease" (PDF). Stroke. 21 (12 Suppl): IV149 – IV151. PMID 2260140.
- ↑ Endo S, Akiyama K (November 1996). "[Thromboxane A2 receptor antagonist in asthma therapy]". Nihon Rinsho. Japanese Journal of Clinical Medicine (in Japanese). 54 (11): 3045–3048. PMID 8950952.
- ↑ Rizk JG, Kalantar-Zadeh K, Mehra MR, Lavie CJ, Rizk Y, Forthal DN (September 2020). "Pharmaco-Immunomodulatory Therapy in COVID-19". Drugs. 80 (13): 1267–1292. doi:10.1007/s40265-020-01367-z. PMC 7372203. PMID 32696108.
- ↑ "Synthesis of (R)-Ramatroban". Synfacts. 8 (08): 0822–0822. August 2012. doi:10.1055/s-0032-1316596.
- ↑ Busto E, Gotor-Fernández V, Gotor V (May 2012). "Asymmetric chemoenzymatic synthesis of ramatroban using lipases and oxidoreductases". The Journal of Organic Chemistry. 77 (10): 4842–8. doi:10.1021/jo300552v. PMID 22515546.
- ↑ Rosentreter U, Böshagen H, Seuter F, Perzborn E, Fiedler VB (December 1989). "Synthesis and absolute configuration of the new thromboxane antagonist (3R)-3-(4-fluorophenylsulfonamido)-1,2,3,4-tetrahydro-9-carbazolepropan oic acid and comparison with its enantiomer". Arzneimittel-Forschung. 39 (12): 1519–21. PMID 2624597.
- ↑ Gardner, P. D., Haynes, G. R., Brandon, R. L. (October 1957). "Formation of Dieckmann Reaction Products under Acyloin Conditions. Competition of the Two Reactions". The Journal of Organic Chemistry. 22 (10): 1206–1210. doi:10.1021/jo01361a021.
- ↑ Horst Bohagen, et al. U.S. patent 5,374,647 (1994 to Bayer AG).
External links
- (in Japanese) Baynas Tablets Prescribing Information