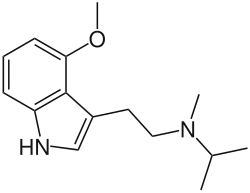
4-MeO-MiPT
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 4-OMe-MiPT; 4-Methoxy-N-methyl-N-isopropyltryptamine |
| Routes of administration | Oral[1] |
| Drug class | Non-selective serotonin receptor agonist; Serotonin 5-HT2A receptor agonist; Serotonergic psychedelic; Hallucinogen; Serotonin reuptake inhibitor |
| ATC code |
|
| Pharmacokinetic data | |
| Onset of action | 20–40 minutes[1] |
| Duration of action | 4–6 hours[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H22N2O |
| Molar mass | 246.354 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 80 to 81 °C (176 to 178 °F) |
SMILES
| |
InChI
| |
| (verify) | |
4-MeO-MiPT, also known as 4-methoxy-N-methyl-N-isopropyltryptamine, is a lesser-known psychedelic drug of the tryptamine and 4-methoxytryptamine families.[1] It is the 4-methoxy analogue of MiPT and the O-methyl ether of 4-HO-MiPT.[1] The drug is taken orally.[1]
It acts as a serotonin reuptake inhibitor and as a non-selective serotonin receptor agonist, including of the serotonin 5-HT2A receptor.[2][3] The drug produces psychedelic-like effects in animals.[3]
4-MeO-MiPT was first described by David Repke and Alexander Shulgin and colleagues in 1985.[4] It was subsequently further described by Shulgin in his 1997 book TiHKAL (Tryptamines I Have Known And Loved).[1] The drug was reported as a novel designer drug by 2016.[5] Very little data exists about the pharmacological properties, metabolism, and toxicity of 4-MeO-MiPT.[1][2]
Use and effects
Shulgin found the effective dose to be 20 to 30 mg (or ~0.4 mg/kg body weight of subject) orally; the onset between ingestion and the first noticeable effects was 20 to 40 minutes, with a listed duration of 4 to 6 hours.[1][4] The effects were significantly milder than those of 4-HO-MiPT, with 4-MeO-MiPT producing erotic-enhancing effects, and few of the visuals common with tryptamines.[1]
Interactions
Pharmacology
Pharmacodynamics
| Target | Affinity (Ki, nM) |
|---|---|
| 5-HT1A | 347–731 (Ki) 1,490 (EC50) 99% (Emax) |
| 5-HT2A | 178 (Ki) 376a (EC50) 63% (Emax) |
| 5-HT2C | 510 (Ki) 120a (EC50) 82%a (Emax) |
| SERT | 38 (Ki) 53–57 (IC50) |
| Notes: The smaller the value, the more avidly the drug interacts with the site. Footnotes: a = Stimulation of IP1 formation. Sources: [2][3] | |
4-MeO-MiPT acts as a serotonin reuptake inhibitor (SRI) and non-selective serotonin receptor agonist, including of the serotonin 5-HT1A, 5-HT2A, 5-HT2C receptors.[2][3] Affinities towards receptors outside of the serotonin receptor family have not yet been assessed.[2][3]
Increased extracellular concentrations of serotonin, resulting from SERT blockade, similarly may compete at the serotonin 5-HT2A receptor, altering or blunting effects mediated by this receptor, which could potentially explain anecdotal reports of subjective effects being dose-dependently milder than that of 4-HO-MiPT or 5-MeO-MiPT.[1][2] This profile makes 4-MeO-MiPT a potential candidate for elucidating the role of SERT blockade in the mechanisms underlying serotonergic psychedelic action.[2][3]
The drug induces the head-twitch response, a behavioral proxy of psychedelic effects, in rodents.[3] Its potency for inducing the head-twitch response in mice is similar to that of 4-HO-MiPT and 4-AcO-MiPT, but the efficacy for doing so is markedly lower: 34 head twitches versus around 80 head twitches per 30 minutes for the aforementioned compounds.[3]
Chemistry
4-MeO-MiPT is synthetic derivative of the substituted tryptamine and 4-methoxytryptamine families.[1][2] It is the 4-methoxy analogue of N-methyl-N-isopropyltryptamine (MiPT) and the O-methyl ether of 4-HO-MiPT.[1][2]
Synthesis
The chemical synthesis of 4-MeO-MiPT has been described.[1][4]
Analogues
Analogues of 4-MeO-MiPT include N-methyl-N-isopropyltryptamine (MiPT), 4-methoxytryptamine (4-MeO-T), 4-MeO-DiPT, 4-MeO-DMT, 4-HO-MiPT, 4-AcO-MiPT, 5-MeO-MiPT, 5-MeO-DMT, and psilocin (4-HO-DMT), among others.[1][2]
History
4-MeO-MiPT was first described in the scientific literature by David Repke and Alexander Shulgin and colleagues in 1985.[4] Subsequently, it was described in greater detail by Alexander Shulgin in his 1997 book TiHKAL (Tryptamines I Have Known Loved) as entry #39.[1] The pharmacology of 4-MeO-MiPT was studied and described in the 2020s.[2][3] It was reported as a novel designer drug by at least 2016.[5]
See also
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Shulgin A, Shulgin A (September 1997). TiHKAL: The Continuation. Berkeley, California: Transform Press. ISBN 0-9630096-9-9. OCLC 38503252. http://www.erowid.org/library/books_online/tihkal/tihkal39.shtml
- 1 2 3 4 5 6 7 8 9 10 11 Kozell LB, Eshleman AJ, Swanson TL, Bloom SH, Wolfrum KM, Schmachtenberg JL, et al. (April 2023). "Pharmacologic Activity of Substituted Tryptamines at 5-Hydroxytryptamine (5-HT)2A Receptor (5-HT2AR), 5-HT2CR, 5-HT1AR, and Serotonin Transporter". The Journal of Pharmacology and Experimental Therapeutics. 385 (1): 62–75. doi:10.1124/jpet.122.001454. PMC 10029822. PMID 36669875.
- 1 2 3 4 5 6 7 8 9 Glatfelter G, Walther D, Partilla J, Chadeayne AR, Manke DR, Baumann MH (2024-06-01). "Pharmacological profiles and psychedelic-like effects of 4-hydroxy-, 4-acetoxy-, and 4-methoxy-N- methyl- N- isopropyltryptamine". The Journal of Pharmacology and Experimental Therapeutics. 389: 281. doi:10.1124/jpet.281.923160. ISSN 0022-3565.
- 1 2 3 4 Repke DB, Grotjahn DB, Shulgin AT (July 1985). "Psychotomimetic N-methyl-N-isopropyltryptamines. Effects of variation of aromatic oxygen substituents". Journal of Medicinal Chemistry. 28 (7): 892–896. doi:10.1021/jm00145a007. PMID 4009612.
- 1 2 Taschwer M, Ebner E, Schmid M (2016). "Test purchase of new synthetic tryptamines via the Internet: Identity check by GC-MS and separation by HPLC" (PDF). Journal of Applied Pharmaceutical Science: 028–034. doi:10.7324/JAPS.2016.600105. ISSN 2231-3354. Retrieved 24 October 2025.