Α,N,N,O-TeMS
 | |
| Clinical data | |
|---|---|
| Other names | 5-Methoxy-α-methyl-N,N-dimethyltryptamine; 5-Methoxy-α,N,N-trimethyltryptamine; α,N,N,O-Tetramethylserotonin; 5-MeO-α,N,N-TMT; 5-MeO-α-methyl-DMT; 5-MeO-α-Me-DMT; α,N,N,O-TeMS |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
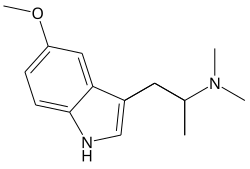
| Formula | C14H20N2O |
| Molar mass | 232.327 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
α,N,N,O-Tetramethylserotonin (α,N,N,O-TeMS), also known as 5-methoxy-α,N,N-trimethyltryptamine (5-MeO-α,N,N-TMT), is a synthetic compound of the tryptamine, α-alkyltryptamine, and 5-methoxytryptamine families.[1][2][3] It is the combined derivative of α-methyltryptamine (αMT) and 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT).[1][2][3]
Use and effects
α,N,N,O-TeMS was described by Alexander Shulgin in his book TiHKAL (Tryptamines I Have Known and Loved) as a putative psychedelic drug.[1] However, Shulgin does not appear to have ever synthesized or assayed it.[1] As such, α,N,N,O-TeMS's effects, dose, and duration are all unknown.[1]
α,N,N,O-TeMS is the N,N-dimethylated derivative of 5-MeO-αMT (α,O-DMS; 5-methoxy-α-methyltryptamine) and the N-methylated derivative of 5-MeO-α,N-DMT (α,N,O-TMS; 5-methoxy-α,N-dimethyltryptamine).[1][2][3] 5-MeO-α,N-DMT is less potent and long-lasting than 5-MeO-αMT, with 5-MeO-α,N-DMT having a dose range of 10 to 20 mg and a duration of 6 to 8 hours versus 5-MeO-αMT having a dose range of 2.5 to 4.5 mg and a duration of 12 to 18 hours.[1][4] Similarly, α,N-DMT (α,N-dimethyltryptamine) is less potent than αMT, with doses of 50 to 100 mg for α,N-DMT and doses of 15 to 30 mg for αMT.[4] Hence, of potential relevance to α,N,N,O-TeMS, it appears that N-methylation of α-alkyltryptamines may reduce their activity, by about 3- or 4-fold.[1][4]
Interactions
History
α,N,N,O-TeMS was first described in the literature, specifically in TiHKAL, by 1997.[1] It is known to have been made at Edgewood Arsenal, but the facility never published anything on the compound.[2] α,N,N,O-TeMS does not appear to have been otherwise described in the literature.[2][3]
See also
- Substituted α-alkyltryptamine
- α,N,N-Trimethyltryptamine (α,N,N-TMT)
- Zalsupindole ((R)-5-methoxy-α,N,N-trimethylisotryptamine; (R)-iso-5-MeO-α,N,N-TMT) – a close isotryptamine analogue of α,N,N,O-TeMS
References
- 1 2 3 4 5 6 7 8 9 Shulgin A (1997). Tihkal: The Continuation. Transform Press. #55. α,N,O-TMS. ISBN 978-0-9630096-9-2. Retrieved 17 August 2024.
With this, now, as a challenge, predict for me the potency of α,N,N,O-tetramethylserotonin. Here is a compound that has not been yet synthesized, but which carries the second N-methyl group (yet closer to DMT at the nitrogen atom and probably more potent) and yet a structural kiss of death (as to potency) in the MDA/MDMA world. Will it be up? Will it be down? I am afraid that the "make 'em and taste 'em" procedure is the only one that I can trust.
- 1 2 3 4 5 "N,N-Dimethyl-5-methoxy-alpha-methyltryptamine". PubChem. Retrieved 10 January 2025.
8 Literature: 8.1 Consolidated References: U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals., NX#12314.
- 1 2 3 4 "5-MeO-α-methyl-DMT". Isomer Design. 11 November 2024. Retrieved 26 November 2024.
- 1 2 3 Shulgin AT (2003). "Basic Pharmacology and Effects". In Laing RR (ed.). Hallucinogens: A Forensic Drug Handbook. Forensic Drug Handbook Series. Elsevier Science. pp. 67–137 (102). ISBN 978-0-12-433951-4.