MEDA
 | |
| Clinical data | |
|---|---|
| Other names | 3-Methoxy-4,5-ethylenedioxyamphetamine; 5-Methoxy-3,4-ethylenedioxyamphetamine; 5-Methoxy-EDA; 5-MeO-EDA |
| Routes of administration | Oral[1] |
| ATC code |
|
| Pharmacokinetic data | |
| Duration of action | Unknown[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
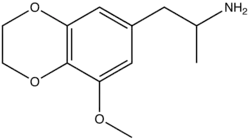
| Formula | C12H17NO3 |
| Molar mass | 223.272 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
MEDA, also known as 3-methoxy-4,5-ethylenedioxyamphetamine or as 5-methoxy-EDA, is a chemical compound of the phenethylamine, amphetamine, and EDxx families.[1][2] It is the EDxx analogue of MMDA (5-methoxy-MDA).[1][2][3] The compound was first synthesized by Alexander Shulgin.[1][2] In his book PiHKAL, the minimum dose is listed as 200 mg, and the duration as unknown.[1][2] MEDA produced few to no effects.[1][2] Very little data exists about the pharmacological properties, metabolism, and toxicity of MEDA.[1][2] MEDA was first described in the scientific literature by Shulgin in 1964.[3][1][2]
See also
- Substituted ethylenedioxyphenethylamine
- MTDA (5-methoxy-TDA)
References
- 1 2 3 4 5 6 7 8 9 MEDA entry in PiHKAL
- 1 2 3 4 5 6 7 Shulgin AT (2003). "Basic Pharmacology and Effects". In Laing RR (ed.). Hallucinogens: A Forensic Drug Handbook. Forensic Drug Handbook Series. Elsevier Science. pp. 67–137. ISBN 978-0-12-433951-4. Archived from the original on 13 July 2025.
- 1 2 Shulgin AT (March 1964). "3-Methoxy-4,5-methylenedioxy Amphetamine, a New Psychotomimetic Agent". Nature. 201 (4924): 1120–1121. Bibcode:1964Natur.201.1120S. doi:10.1038/2011120a0. PMID 14152788. Archived from the original on 2025-07-12.