3-Hydroxy-N,N-dimethylphenethylamine
 | |
| Clinical data | |
|---|---|
| Other names | LSM-6; LSM6 |
| Routes of administration | Unspecified[1] |
| Drug class | Adrenergic and serotonergic agent[2] |
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
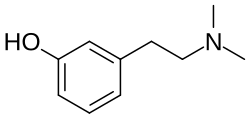
| Formula | C10H15NO |
| Molar mass | 165.236 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
3-Hydroxy-N,N-dimethylphenethylamine (developmental code name LSM-6) is a drug of the phenethylamine family which was under development for the treatment of mood disorders but was never marketed.[1][2] It is the N,N-dimethylated derivative of meta-tyramine (3-hydroxyphenethylamine).[3] The drug is a naturally occurring constituent of Limacia scanden Lour. and is described as an adrenergic and serotonergic agent.[1][2] It may act as a monoamine releasing agent and/or reuptake inhibitor.[2] LSM-6 has sympathomimetic effects.[2] It was being developed in the 1990s in Malaysia.[1] The drug reached the preclinical research stage of development prior to its discontinuation.[1][2]
See also
- List of investigational antidepressants
- Trichocereine (3,4,5-trimethoxy-N,N-dimethylphenethylamine)
- Macromerine (β-hydroxy-3,4-dimethoxy-N,N-dimethylphenethylamine)
- Dimethylamphetamine (N,N-dimethylamphetamine)
- Gepefrine (3-hydroxyamphetamine)
- Pholedrine (4-hydroxy-N-methylamphetamine)
- 4-Hydroxyamphetamine (norpholedrine)
References
- 1 2 3 4 5 "LSM 6 - AdisInsight". adisinsight.springer.com.
- 1 2 3 4 5 6 Hwi KK, Lay WB (September 1998). "Pharmacological, electrophysiological and toxicity studies of Limacia scanden Lour. (Menispermaceae)". J Ethnopharmacol. 62 (2): 137–148. doi:10.1016/s0378-8741(98)00045-2. PMID 9741886.
- ↑ "Phenol, 3-(2-dimethylamino)ethyl-". PubChem. Retrieved 1 March 2025.