Trazium
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
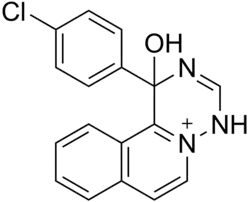
| Formula | C17H13ClN3O |
| Molar mass | 310.76 g·mol−1 |
Trazium (EGYT-3,615) is an antidepressant drug which was never marketed.[1] It has psychostimulant-like effects and its actions appear to be mediated by the dopaminergic and adrenergic systems.[2] It was formulated as a salt with ethanesulfonic acid and given the generic name trazium esilate (INN).
Synthesis
Precursors:[3][4][5] Background literature:[6][7][8][9]
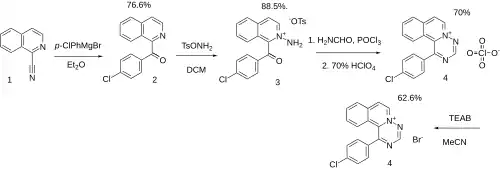
Ex 5: The Grignard reaction between para-chlorophenylmagnesium bromide and 1-cyanoisoquinoline [1198-30-7] (1) gave 1-(4-chlorobenzoyl)isoquinoline, PC12243105 (2). The reaction of this with O-tosylhydroxylamine [52913-14-1] gives (2-aminoisoquinolin-2-ium-1-yl)-(4-chlorophenyl)methanone;4-methylbenzenesulfonate, PC23311140 (3). The reaction of this a mixture of formamide and phosphoryl chloride gave the ring. The addition of perchloric acid led to 1-(4-Chlorophenyl)[1,2,4]triazino[6,1-a]isoquinolin-5-ium perchlorate [82319-70-8] (4). The reaction of this with triethylammonium bromide is said to give (4).
Patent:[10]
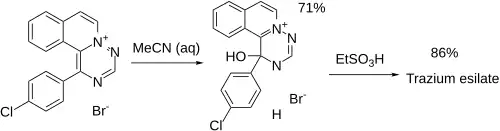
Ex 1: 1-(4-chlorophenyl)-as-triazino[6,1-a]isoquinolinium-bromide [82319-85-5] (1) is reacted with an aqueous solution of acetonitrile to give the title compound. Ex 2: Treatment with ethanesulfonic acid in acetonitrile then prepares the esilate salt.
References
- ↑ David J. Triggle (1996). Dictionary of Pharmacological Agents. Boca Raton: Chapman & Hall/CRC. ISBN 0-412-46630-9.
- ↑ Gyertyán I, Petöcz L, Bajnógel J, et al. (July 1989). "Possible involvement of the dopaminergic system in the mode of action of the potential antidepressant trazium esilate". Arzneimittel-Forschung. 39 (7): 775–81. PMID 2551306.
- ↑ DE3128386 idem Sandor Batori, 8 More », US4419355 (1983 to Egyt Gyogyszervegyeszeti Gyar).
- ↑ Hungarian Patent application Ser. No. 3243/83. idem Andras Messmer, 7 More », US4753938 (1988 to Richter Gedeon Vegyeszeti Gyar Rt.).
- ↑ , US4697013 ().
- ↑ Kakehi, Akikazu; Ito, Suketaka; Uchiyama, Kenji; Konno, Yoshiaki (1976). "SYNTHESIS AND CHARACTERIZATION OF 1-IMIDOYLIMINOPYRIDINIUM N-YLIDES". Chemistry Letters. 5 (5): 413–414. doi:10.1246/cl.1976.413.
- ↑ Kakehi, Akikazu; Ito, Suketaka; Uchiyama, Kenji; Konno, Yoshiaki; Kondo, Kenji (1977). "Thermolysis and photolysis of various N-imidoyliminopyridinium ylides". The Journal of Organic Chemistry. 42 (3): 443–448. doi:10.1021/jo00423a012.
- ↑ Neunhoeffer, Hans; Lehmann, Bernd; Ewald, Herbert (1977). "Zur Chemie der 1,2,4-Triazine, VIII. Struktur eines Reaktionsproduktes von 3-(p-Tolyl)-1,2,4-triazinen mit Acetylendicarbonsäure-dimethylester". Justus Liebigs Annalen der Chemie. 1977 (9): 1421–1428. doi:10.1002/jlac.197719770903.
- ↑ Ewald, Herbert; Lehmann, Bernd; Neunhoeffer, Hans (1977). "Cycloadditionen mit Azabenzolen, XII. Reaktionen von 1,2,4-Triazinen mit Acetylendicarbonsäuredimethylester". Justus Liebigs Annalen der Chemie. 1977 (10): 1718–1724. doi:10.1002/jlac.197719771018.
- ↑ BE900598 idem Andras Messmer, Sandor Batori, Gyorgy Hajos, Pal Benko, U.S. patent 4,602,018 (1986 to Egyt Gyogyszevegyeszeti Gyar).