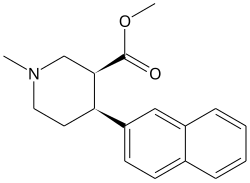
N,O-Dimethyl-4-(2-naphthyl)piperidine-3-carboxylate
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H21NO2 |
| Molar mass | 283.371 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
| (verify) | |
N,O-Dimethyl-4β-(2-naphthyl)piperidine-3β-carboxylate (DMNPC) is a piperidine based stimulant drug which is synthesised from arecoline. It is similar to nocaine in chemical structure, and has two and a half times more activity than cocaine as a dopamine reuptake inhibitor. However it is also a potent serotonin reuptake inhibitor, with similar affinity to fluoxetine.[1]
DMNPC has four stereoisomers, each of which has different binding affinities, with the 3S,4S enantiomer having the highest overall activity. The 3R,4S enantiomer demonstrates the highest selectivity for 5-HTT.
| Binding affinities (Ki) | ||||||
| Stereochemistry | 5HT | DA | NE | |||
| 3S,4S | 7.6 | 21 | 34 | |||
| 3R,4S | 42 | 947 | 241 | |||
| 3R,4R | 192 | 87 | 27 | |||
| 3S,4R | 12 | 271 | 38 | |||
In animal studies, DMNPC exhibits similar potency as fluoxetine, but with greater activity for DAT and NET. N-Demethylation of DMNPC has shown to produce a 3-fold increase in potency for 5-HTT.[1]
Synthesis
A racemic mixture of DMNPC can be synthesized from freebase arecoline in a grignard reaction with 2-naphthylmagnesium bromide. Further reactions and separation methods can be used to produce enantiomerically pure products.[1]
A substantially simpler method that ablates the carbomethoxy ester substituent has been demonstrated by D. Koch.[2]
See also
References
- 1 2 3 Tamiz AP, Zhang J, Flippen-Anderson JL, Zhang M, Johnson KM, Deschaux O, et al. (March 2000). "Further SAR Studies of Piperidine-Based Analogues of Cocaine. 2. Potent Dopamine and Serotonin Reuptake Inhibitors". Journal of Medicinal Chemistry. 43 (6): 1215–22. doi:10.1021/jm9905561. PMID 10737754.
- ↑ U.S. patent 6,303,627