Strongyloidiasis
| Strongyloidiasis | |
|---|---|
| Other names: Disseminated strongyloidiasis[1], Threadworm infection[2] | |
  | |
| |
| Specialty | Infectious disease |
| Symptoms | Abdominal pain, diarrhea, weight loss[4] |
| Complications | Hyperinfection syndrome[4] |
| Causes | Strongyloides stercoralis[4] |
| Risk factors | Immunocompromisation[4] |
| Diagnostic method | Serology, stool tests[4] |
| Differential diagnosis | Intestinal obstruction,Loffler syndrome,ARDS,peritonitis[4] |
| Treatment | Ivermectin[4] |
Strongyloidiasis is a human parasitic disease caused by the nematode called Strongyloides stercoralis, or sometimes the closely related S. fülleborni. These helminths belong to a group of nematodes called roundworms. These intestinal worms can cause a number of symptoms in people, principally skin symptoms, abdominal pain, diarrhea and weight loss, but also many other specific and vague symptoms in disseminated disease, and severe life-threatening conditions through hyperinfection. In some people, particularly those who require corticosteroids or other immunosuppressive medication, Strongyloides can cause a hyperinfection syndrome that can lead to death if untreated. The diagnosis is made by blood and stool tests. The medication ivermectin is widely used to treat strongyloidiasis.[4][5][6][7]
Strongyloidiasis is a type of soil-transmitted helminthiasis. Low estimates postulate it to affect 30–100 million people worldwide,[8] mainly in tropical and subtropical countries, while higher estimates conservatively extrapolate that infection is upwards to or above 370 million people.[9] It belongs to the group of neglected tropical diseases, and worldwide efforts are aimed at eradicating the infection.[10]
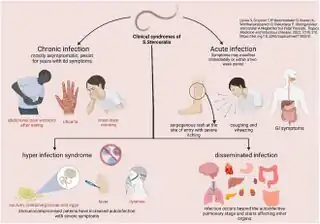
Signs and symptoms
Strongyloides infection occurs in several forms. As the infection continues and the larvae matures, there may be respiratory symptoms (Löffler's syndrome). The infection may then become chronic with mainly digestive symptoms. On reinfection from the skin to the lungs and finally to the small intestine, there may be respiratory, skin and digestive symptoms. Finally, the hyperinfection syndrome causes symptoms in many organ systems, including the central nervous system.[11][12][4]
Uncomplicated
Frequently asymptomatic, gastrointestinal system symptoms include abdominal pain and diarrhea and/or conversely constipation. Pulmonary symptoms can occur during pulmonary migration of the filariform larvae. Pulmonary infiltrate may be present through radiological investigation. Dermatologic manifestations include urticarial rashes in the buttocks and waist areas as well as larva currens. Eosinophilia is generally present. Strongyloidiasis can become chronic and then become completely asymptomatic.[4][13]
Disseminated
Disseminated strongyloidiasis occurs when patients with chronic strongyloidiasis become immunosuppressed. There is a distinction to be made between dissemination and hyperinfection. It is mainly a semantic distinction. There can be mild dissemination where the worm burden is relatively lower yet causes insidious symptoms, or extreme dissemination that the term hyperinfection is used to describe. Thus hyperinfection of varying levels of severe dissemination may present with abdominal pain, distension, shock, pulmonary and neurologic complications, sepsis, haemorrhage, malabsorption, and depending on the combination, degree, number, and severity of symptoms, is potentially fatal. The worms enter the bloodstream from the bowel wall, simultaneously allowing entry of bowel bacteria such as Escherichia coli. This may cause symptoms such as sepsis,[14] and the bacteria may spread to other organs where they may cause localized infection such as meningitis.[15] Dissemination can occur many decades after the initial infection[16] and has been associated with high dose corticosteroids, organ transplant, any other instances and causes of immunosuppression.[17][18]
Cause
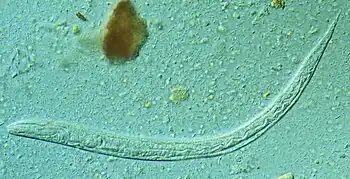
Strongyloides stercoralis is a soil-transmitted helminth.Contagiousness via textiles, unlike Enterobius vermicularis, is unfounded. It has rarely been transmitted through organ transplantation.[4][19] Married Vietnam War veterans who were infected, yet never developed significant hyperinfection, lived for multiple decades with non-debilitating disseminated infection, without treatment, with wives who failed to ever contract infection.[20] Contraction occurs overwhelmingly from skin exposure to any contaminated soil, contaminated potting soil, contaminated waters, lack of sanitation, or environmental factors as potential vectors. Nearly never to extraordinarily very rarely documented is transmission from person to person, other than closeness of contact to the productive coughing of a very ill hyperinfected individual. It has been shown possible to occur in that situation, or potentially other similar scenarios, it is speculated via pulmonary secretions of a direly hyperinfected individual. In which case treatment for others may be indicated, if deemed necessary by proximity, symptoms, precautions, probable exposures to the same vectors, or through screening of serology and stool samples, until infection is eradicated.[21]
Mechanism
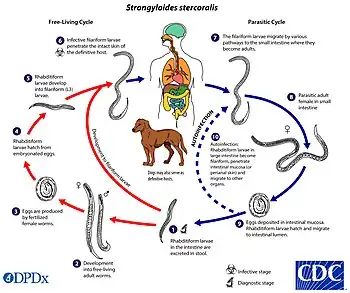
In terms of the mechanism that causes Strongyloidiasis we find that the larvae can develop into free-living adult males and females. These adults reproduce sexually, ready to continue the cycle. They actively penetrate the skin of a human host, often through bare feet.The larvae enter the bloodstream and travel to the lungs.From the lungs, they migrate to the trachea and are swallowed.In the intestine, the larvae mature into adult female worms.The females reproduce through a process called parthenogenesis, laying eggs that hatch into larvae in the intestine.Larvae are then either excreted or larvae develop into a new generation of infective larvae within the host, leading to autoinfection.[4][22]
Diagnosis
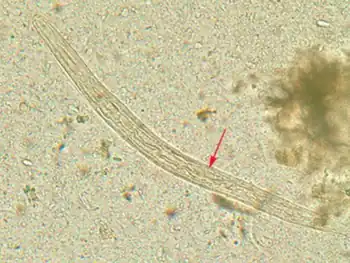
Diagnosis rests on the microscopic identification of larvae (rhabditiform and occasionally filariform) in the stool or duodenal fluid. Examination of many samples may be necessary, and not always sufficient, because direct stool examination is relatively insensitive, with a single sample only able to detect larvae in a small percent of cases. It can take 4 weeks from initial infection to the passage of larvae in the stool.The stool can be examined in wet mounts via a variety of methods[23][4][24]
Culture techniques are the most sensitive, but are not routinely available in the West. In the UK, culture is available at either of the Schools of Tropical Medicine in Liverpool or London. Direct examination must be done on stool that is freshly collected and not allowed to cool down, because hookworm eggs hatch on cooling and the larvae are very difficult to distinguish from Strongyloides.Finding Strongyloides in the stool is negative in up to 70% of tests. It is important to undergo frequent stool sampling as well as duodenal biopsy if a bad infection is suspected. The duodenal fluid can be examined using techniques such as the Enterotest string or duodenal aspiration. Given the poor ability of stool examination to diagnose Strongyloides, detecting antibodies by ELISA can be useful.[25][4][26]
Serology can cross-react with other parasites, remain positive for years after successful treatment or be falsely negative in immunocompromised patients.[23][27] Infected patients will also often have an elevated eosinophil count, with an average of absolute eosinophil count of 1000 in one series.[28] It would be greatly useful to have significant advances in the sensitivity of the means of diagnosis, as it would also solve the challenging problem of proof of cure. If definitive diagnosis is solved then it stands to reason that proof of cure becomes easily realizable.[29]
.jpg)
Differential diagnosis
In terms of the DDx in the affected individual we find the following:[4]
- Intestinal obstruction
- Loffler syndrome
- ARDS
- Peritonitis
- Pneumonia
- Pneumonitis
Screening
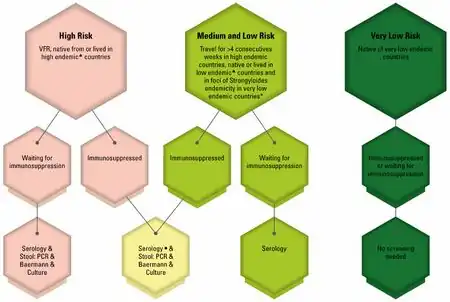
It is often recommended that patients being started on immunosuppression be screened for chronic strongyloidiasis; however, in developed countries, the prevalence of chronic strongyloidiasis is very small, so screening is usually not cost-effective, except in endemic areas.[31][30]
Screening should be performed before administering steroids for infection in even remotely susceptible individuals in order to prevent escalating the infection is advised. As not doing so in certain cohorts can have high mortality rates from inadvertently caused hyperinfection via immunosuppression of application of certain steroids. Thus caution with respect to iatrogenic risks is crucial to avoiding adverse consequences in treatment.[32][30]
People with high exposure to Strongyloides stercoralis may mitigate the risk of strongyloidiasis hyperinfection associated with corticosteroid treatment, with the presumptive use of ivermectin. Such hyperinfection has been a particular concern during the COVID-19 pandemic because of the use of corticosteroids for treatment of COVID-19 symptoms. The CDC and other international bodies recommend the use of ivermectin for refugees from areas which have a risk of strongyloidiasis.[33]
Treatment
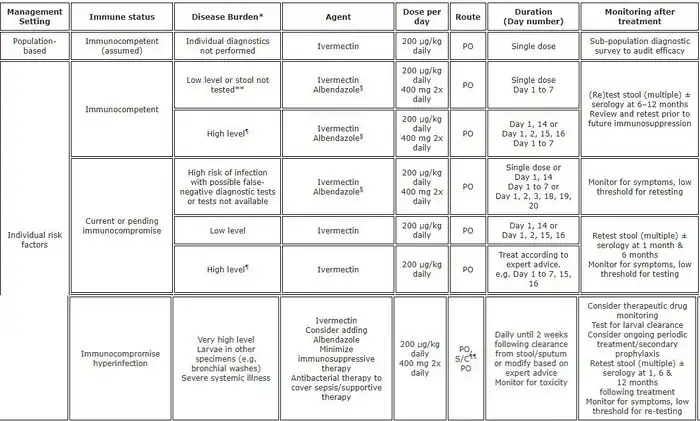
The consensus drug of choice for the treatment of uncomplicated strongyloidiasis is ivermectin. However, even if it is considered the main drug of choice, recent studies have illustrated the challenges in ivermectin curing strongyloidiasis.[35] Ivermectin does not kill the Strongyloides larvae, only the adult worms, therefore repeat dosing may be necessary to properly eradicate the infection. There is an auto-infective cycle of roughly two weeks in which ivermectin should be re-administered; however, additional dosing may still be necessary as it will not kill Strongyloides in the blood or larvae deep within the bowels or diverticula.[36] Other drugs that can be effective are albendazole and thiabendazole (25 mg/kg twice daily for 5 days—400 mg maximum (generally)).[18] All patients who are at risk of disseminated strongyloidiasis should be treated. The optimal duration of treatment for patients with disseminated infections is not clear.[17]
Treatment of strongyloidiasis can be difficult and if ceasing treatment before being entirely cleared Strongyloides via the autoinfective cycle has been known to live in individuals for decades;[37] even after initial or inadequate sustained treatment. Continued treatment, blood and stool monitoring thus may be necessary even if symptoms temporarily resolve. As cited earlier, due to the fact that some infections are insidiously asymptomatic, and relatively expensive bloodwork is often inconclusive via false-positives or false-negatives,[38] just as stool samples can be unreliable in diagnoses,[39] there is yet unfortunately no real gold standard for proof of cure, mirroring the lack of an efficient and reliable methodology of diagnosis.[11][29][40] An objective eradication standard for strongyloidiasis is elusive given the high degree of suspicion needed to even begin treatment, the sometimes difficulty of the only definitive diagnostic criteria of detecting and isolating larvae or adult Strongyloides, the importance of early diagnosis, particularly before steroid treatments,[41] and the very wide variability and exclusion/inclusion of differing collections of diffuse symptoms. Disregarding mis-ascribing bonafide delusional parasitosis disorders,[42][43][44] strongyloidiasis should be more well known among medical professionals and have serious consideration for broad educational campaigns in effected geographic locales both within the semi-tropical developed world and otherwise, as well as in the tropical developing world where, among many other neglected tropical diseases, it is endemic.[7][45]
There are conflicting reports on effective drug treatments. Ivermectin ineffectiveness and rising drug resistance has been documented.[46] Albendazole is noted by the WHO as being the least effective.[47] Thiabendazole can have severe side effects and is unavailable in many countries.[48]
Prognosis
In terms of the prognosis we find that acute and chronic infections is positive, when treatment occurs. But in hyperinfection syndrome and disseminated infection, 70 to 100 percent is the current mortality rate[4]
It is important to note that there is not necessarily any eosinophilia in the disseminated disease. Absence of eosinophilia in an infection limited to the gastrointestinal tract may indicate poor prognosis.[49]
Epidemiology
Low estimates postulate it to affect 30–100 million people worldwide,[8] mainly in tropical and subtropical countries, while higher estimates conservatively extrapolate that infection is upwards to or above 370 million people.[9]
It belongs to the group of neglected tropical diseases, and worldwide efforts are aimed at eradicating the infection.[10]
History

The disease was first recognized in 1876 by the French physician Louis Alexis Normand, working in the naval hospital in Toulon; he identified the adult worms, and sent them to Arthur Réné Jean Baptiste Bavay, chief inspector for health, who observed that these were the adult forms of the larvae found in the stool.[52]
In 1883 the German parasitologist Rudolf Leuckart made initial observations on the life cycle of the parasite, and Belgian physician Paul Van Durme (building on observations by the German parasitologist Arthur Looss) described the mode of infection through the skin. The German parasitologist Friedrich Fülleborn described autoinfection and the way by which strongyloidiasis involves the intestine. Interest in the condition increased in the 1940s when it was discovered that those who had acquired the infection abroad and then received immunosuppression developed hyperinfestation syndrome.[52] During the 1940s, the treatment of choice was enteric coated tablets of 60 mg gentian violet, three times daily, for 16 days.[53]
References
- ↑ "Strongyloidiasis". GARD. Archived from the original on 30 April 2021. Retrieved 23 April 2025.
- ↑ "Infection by Strongyloides (Concept Id: C0038463) - MedGen - NCBI". www.ncbi.nlm.nih.gov. Archived from the original on 23 March 2025. Retrieved 18 March 2025.
- ↑ Pillai, Rishvini Kailashnath; Pillai, Roshvin Kailashnath; Illankovan, Vinibha Rajakumari; Kumarasamy, Vinoth; Reddy, Satyanarayana; Gowtham, K.; Dhanasekaran, Muralikrishnan; Subramaniyan, Vetriselvan (December 2024). "Understanding Strongyloides Stercoralis infection and its relationship to chronic alcohol abuse: Understanding pathogenesis and therapeutic strategies". Toxicology Reports. 13: 101754. Bibcode:2024ToxR...1301754P. doi:10.1016/j.toxrep.2024.101754. PMC 11493194. PMID 39434865.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 Mora Carpio, Andres L.; Meseeha, Marcelle (2025). "Strongyloidiasis". StatPearls. StatPearls Publishing. PMID 28613795. Archived from the original on 2025-05-02. Retrieved 2025-03-20.
- ↑ Mahmoud, Adel A. F. (1996). "Strongyloidiasis". Clinical Infectious Diseases. 23 (5): 949–952. doi:10.1093/clinids/23.5.949. ISSN 1058-4838. JSTOR 4459767. PMID 8922784. Retrieved 25 March 2025.
- ↑ Gordon, Catherine A.; Utzinger, Jürg; Muhi, Stephen; Becker, Sören L.; Keiser, Jennifer; Khieu, Virak; Gray, Darren J. (25 January 2024). "Strongyloidiasis". Nature Reviews Disease Primers. 10 (1): 1–16. doi:10.1038/s41572-023-00490-x. ISSN 2056-676X. PMID 38272922. Archived from the original on 16 April 2024. Retrieved 25 March 2025.
- ↑ 7.0 7.1 Buonfrate, Dora; Requena-Mendez, Ana; Angheben, Andrea; Muñoz, Jose; Gobbi, Federico; Van Den Ende, Jef; Bisoffi, Zeno (8 February 2013). "Severe strongyloidiasis: a systematic review of case reports". BMC Infectious Diseases. 13 (1): 78. doi:10.1186/1471-2334-13-78. ISSN 1471-2334. PMID 23394259.
- ↑ 8.0 8.1 Buonfrate D, Formenti F, Perandin F, Bisoffi Z (June 2015). "Novel approaches to the diagnosis of Strongyloides stercoralis infection". Clinical Microbiology and Infection. 21 (6): 543–52. doi:10.1016/j.cmi.2015.04.001. PMID 25887711.
- ↑ 9.0 9.1 Varatharajalu R, Kakuturu R (2016). "Strongyloides stercoralis: Current perspectives". Reports in Parasitology: 23. doi:10.2147/RIP.S75839.
- ↑ 10.0 10.1 "Neglected Tropical Diseases". cdc.gov. June 6, 2011. Archived from the original on 4 December 2014. Retrieved 28 November 2014.
- ↑ 11.0 11.1 Montes M, Sawhney C, Barros N (October 2010). "Strongyloides stercoralis: there but not seen". Current Opinion in Infectious Diseases. 23 (5): 500–4. doi:10.1097/QCO.0b013e32833df718. PMC 2948977. PMID 20733481.
- ↑ Marcos LA, Terashima A, Dupont HL, Gotuzzo E (April 2008). "Strongyloides hyperinfection syndrome: an emerging global infectious disease". Transactions of the Royal Society of Tropical Medicine and Hygiene. 102 (4): 314–8. doi:10.1016/j.trstmh.2008.01.020. PMID 18321548.
- ↑ "Strongyloidiasis". DermNet®. 26 October 2023. Archived from the original on 25 January 2025. Retrieved 27 March 2025.
- ↑ Ghoshal UC, Ghoshal U, Jain M, Kumar A, Aggarwal R, Misra A, Ayyagari A, Naik SR (December 2002). "Strongyloides stercoralis infestation associated with septicemia due to intestinal transmural migration of bacteria". Journal of Gastroenterology and Hepatology. 17 (12): 1331–3. doi:10.1046/j.1440-1746.2002.02750.x. PMID 12423282. S2CID 10004323.
- ↑ Graeff-Teixeira C, da Silva AC, Yoshimura K (April 2009). "Update on eosinophilic meningoencephalitis and its clinical relevance". Clinical Microbiology Reviews. 22 (2): 322–48, Table of Contents. doi:10.1128/CMR.00044-08. PMC 2668237. PMID 19366917.
- ↑ Gill GV, Beeching NJ, Khoo S, Bailey JW, Partridge S, Blundell JW, Luksza AR (June 2004). "A British Second World War veteran with disseminated strongyloidiasis". Transactions of the Royal Society of Tropical Medicine and Hygiene. 98 (6): 382–6. doi:10.1016/j.trstmh.2003.11.002. PMID 15099996.
- ↑ 17.0 17.1 Kramer MR, Gregg PA, Goldstein M, Llamas R, Krieger BP (October 1990). "Disseminated strongyloidiasis in AIDS and non-AIDS immunocompromised hosts: diagnosis by sputum and bronchoalveolar lavage". Southern Medical Journal. 83 (10): 1226–9. doi:10.1097/00007611-199010000-00024. PMID 2218668.
- ↑ 18.0 18.1 Gompels MM, Todd J, Peters BS, Main J, Pinching AJ (March 1991). "Disseminated strongyloidiasis in AIDS: uncommon but important". AIDS. 5 (3): 329–32. doi:10.1097/00002030-199103000-00015. PMID 2059374.
- ↑ "Strongyloidiasis Infection FAQs". United States Centers for Disease Control and Prevention (CDC). 2019-04-23. Archived from the original on 2022-02-12. Retrieved 2022-02-12.
- ↑ Grove DI (August 1982). "Strongyloidiasis: is it transmitted from husband to wife?". The British Journal of Venereal Diseases. 58 (4): 271–2. doi:10.1136/sti.58.4.271. PMC 1046065. PMID 6896668.
- ↑ Czachor JS, Jonas AP (2006). "Transmission of Strongyloides steracolis person to person". Journal of Travel Medicine. 7 (4): 211–2. doi:10.2310/7060.2000.00063. PMID 11003736.
- ↑ "CDC - DPDx - Strongyloidiasis". www.cdc.gov. 30 July 2019. Archived from the original on 24 June 2023. Retrieved 22 March 2025.
- ↑ 23.0 23.1 Segarra-Newnham M (December 2007). "Manifestations, diagnosis, and treatment of Strongyloides stercoralis infection". The Annals of Pharmacotherapy. 41 (12): 1992–2001. doi:10.1345/aph.1K302. PMID 17940124. S2CID 38184274.
- ↑ "Strongyloidiasis Workup". emedicine. Archived from the original on 11 December 2023. Retrieved 27 March 2025.
- ↑ "Strongyloidiasis | CDC Yellow Book 2024". wwwnc.cdc.gov. Archived from the original on 2 April 2025. Retrieved 26 March 2025.
- ↑ Requena-Méndez, Ana; Chiodini, Peter; Bisoffi, Zeno; Buonfrate, Dora; Gotuzzo, Eduardo; Muñoz, José (2013). "The laboratory diagnosis and follow up of strongyloidiasis: a systematic review". PLOS Neglected Tropical Diseases. 7 (1): e2002. doi:10.1371/journal.pntd.0002002. ISSN 1935-2735. PMC 3547839. PMID 23350004.
- ↑ Greiner K, Bettencourt J, Semolic C (2008). "Strongyloidiasis: a review and update by case example". Clinical Laboratory Science. 21 (2): 82–8. PMID 18507302.
- ↑ Nuesch R, Zimmerli L, Stockli R, Gyr N, Christoph Hatz FR (2006). "Imported strongyloidosis: a longitudinal analysis of 31 cases". Journal of Travel Medicine. 12 (2): 80–4. doi:10.2310/7060.2005.12204. PMID 15996452.
- ↑ 29.0 29.1 Mendes T, Minori K, Ueta M, Miguel DC, Allegretti SM (2017). "Strongyloidiasis Current Status with Emphasis in Diagnosis and Drug Research". Journal of Parasitology Research. 2017: 5056314. doi:10.1155/2017/5056314. PMC 5292188. PMID 28210503.
- ↑ 30.0 30.1 30.2 Carnino, Luisa; Schwob, Jean-Marc; Gétaz, Laurent; Nickel, Beatrice; Neumayr, Andreas; Eperon, Gilles (29 November 2021). "A Practical Approach to Screening for Strongyloides stercoralis". Tropical Medicine and Infectious Disease. 6 (4): 203. doi:10.3390/tropicalmed6040203. ISSN 2414-6366. PMC 8704417. PMID 34941659.
- ↑ Requena-Méndez, Ana; Buonfrate, Dora; Gomez-Junyent, Joan; Zammarchi, Lorenzo; Bisoffi, Zeno; Muñoz, José (September 2017). "Evidence-Based Guidelines for Screening and Management of Strongyloidiasis in Non-Endemic Countries". The American Journal of Tropical Medicine and Hygiene. 97 (3): 645–652. doi:10.4269/ajtmh.16-0923. ISSN 1476-1645. PMC 5590585. PMID 28749768.
- ↑ Page W, Speare R (2016). "Chronic strongyloidiasis - Don't look and you won't find". Australian Family Physician. 45 (1): 40–4. PMID 27051986. Archived from the original on 2022-02-18. Retrieved 2022-03-14.
- ↑ "A parasitic infection that can turn fatal with administration of corticosteroids". WHO. 17 December 2021. Archived from the original on 12 December 2021. Retrieved 12 February 2022.
- ↑ Buonfrate, Dora; and Watts, Matthew R. (22 September 2022). "Current pharmacotherapeutic strategies for Strongyloidiasis and the complications in its treatment". Expert Opinion on Pharmacotherapy. 23 (14): 1617–1628. doi:10.1080/14656566.2022.2114829. ISSN 1465-6566.
- ↑ Repetto SA, Ruybal P, Batalla E, López C, Fridman V, Sierra M, Radisic M, Bravo PM, Risso MG, González Cappa SM, Alba Soto CD (May 2018). "Strongyloidiasis Outside Endemic Areas: Long-term Parasitological and Clinical Follow-up After Ivermectin Treatment". Clinical Infectious Diseases. 66 (10): 1558–1565. doi:10.1093/cid/cix1069. PMID 29360939. S2CID 3820549.
- ↑ Strongyloidiasis~treatment at eMedicine
- ↑ "Strongyloidiasis" (PDF). Australian Government. Archived (PDF) from the original on 2016-03-03. Retrieved 2022-02-12.
- ↑ "Strongyloides Antibody, IgG, Serum". Mayo Clinic. Archived from the original on 2018-07-31. Retrieved 2022-02-12.
- ↑ Siddiqui AA, Berk SL (October 2001). "Diagnosis of Strongyloides stercoralis infection". Clinical Infectious Diseases. 33 (7): 1040–7. doi:10.1086/322707. PMID 11528578.
- ↑ Lodh N, Caro R, Sofer S, Scott A, Krolewiecki A, Shiff C (November 2016). "Diagnosis of Strongyloides stercoralis: Detection of parasite-derived DNA in urine". Acta Tropica. 163: 9–13. doi:10.1016/j.actatropica.2016.07.014. PMC 5117362. PMID 27456935.
- ↑ Kassalik M, Mönkemüller K (November 2011). "Strongyloides stercoralis hyperinfection syndrome and disseminated disease". Gastroenterology & Hepatology. 7 (11): 766–8. PMC 3264932. PMID 22298975.
- ↑ "Delusional Parasitosis". Merck Manual. Archived from the original on 2022-01-08. Retrieved 2022-02-12.
- ↑ Prakash J, Shashikumar R, Bhat PS, Srivastava K, Nath S, Rajendran A (January 2012). "Delusional parasitosis: Worms of the mind". Industrial Psychiatry Journal. 21 (1): 72–4. doi:10.4103/0972-6748.110958. PMC 3678185. PMID 23766584.
- ↑ Bak R, Tumu P, Hui C, Kay D, Peng D (October 2008). "A review of delusions of parasitosis, part 2: treatment options". Cutis. 82 (4): 257–64. PMID 19055169.
- ↑ Boulware DR, Stauffer WM, Hendel-Paterson BR, Rocha JL, Seet RC, Summer AP, Nield LS, Supparatpinyo K, Chaiwarith R, Walker PF (June 2007). "Maltreatment of Strongyloides infection: case series and worldwide physicians-in-training survey". The American Journal of Medicine. 120 (6): 545.e1–8. doi:10.1016/j.amjmed.2006.05.072. PMC 1950578. PMID 17524758.
- ↑ Repetto SA, Ruybal P, Batalla E, López C, Fridman V, Sierra M, Radisic M, Bravo PM, Risso MG, González Cappa SM, Alba Soto CD (May 2018). "Strongyloidiasis Outside Endemic Areas: Long-term Parasitological and Clinical Follow-up After Ivermectin Treatment". Clinical Infectious Diseases. 66 (10): 1558–1565. doi:10.1093/cid/cix1069. PMID 29360939. S2CID 3820549.
- ↑ "Strongyloidiasis". World Health Organization. Archived from the original on February 3, 2017.
- ↑ Thiabendazole. 2012. PMID 31643176. Archived from the original on 2019-07-02. Retrieved 2022-02-12.
{{cite book}}:|work=ignored (help) - ↑ Gokhale UA, Pillai GR, Al-Mammari S, Al-Layla D (2010). "Hyperinfection by Strongyloides Stercoralis". Oman Medical Journal. 25 (2): 163–6. doi:10.5001/omj.2010.47 (inactive 23 March 2025). Archived from the original on 14 May 2021. Retrieved 12 February 2022.
{{cite journal}}: CS1 maint: DOI inactive as of March 2025 (link) - ↑ Chan, Abigail Hui En; Kusolsuk, Teera; Watthanakulpanich, Dorn; Pakdee, Wallop; Doanh, Pham Ngoc; Yasin, Azlin Mohd; Dekumyoy, Paron; Thaenkham, Urusa (13 September 2023). "Prevalence of Strongyloides in Southeast Asia: a systematic review and meta-analysis with implications for public health and sustainable control strategies". Infectious Diseases of Poverty. 12 (1): 83. doi:10.1186/s40249-023-01138-4. ISSN 2049-9957. Archived from the original on 12 June 2025. Retrieved 12 June 2025.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ↑ Buonfrate, Dora; Bisanzio, Donal; Giorli, Giovanni; Odermatt, Peter; Fürst, Thomas; Greenaway, Christina; French, Michael; Reithinger, Richard; Gobbi, Federico; Montresor, Antonio; Bisoffi, Zeno (13 June 2020). "The Global Prevalence of Strongyloides stercoralis Infection". Pathogens (Basel, Switzerland). 9 (6): 468. doi:10.3390/pathogens9060468. ISSN 2076-0817. PMC 7349647. PMID 32545787.
- ↑ 52.0 52.1 Cox FE (October 2002). "History of human parasitology". Clinical Microbiology Reviews. 15 (4): 595–612. doi:10.1128/CMR.15.4.595-612.2002. PMC 126866. PMID 12364371.
- ↑ "Clinical Aspects and Treatment of the More Common Intestinal Parasites of Man (TB-33)". Veterans Administration Technical Bulletin 1946 & 1947. 10: 1–14. 1948. Archived from the original on 2022-02-25. Retrieved 2022-02-12.
Further reading
- WHO guideline on preventive chemotherapy for public health control of strongyloidiasis. World Health Organization. 2024. ISBN 978-92-4-009402-4. Archived from the original on 2025-02-08. Retrieved 2025-04-23.
External links
- Strongyloidiasis Archived 2010-10-31 at the Wayback Machine. U.S. Centers for Disease Control and Prevention (CDC)
| Classification | |
|---|---|
| External resources |
![Pooled prevalence of S. stercoralis in Southeast Asia[50]](./_assets_/0c70a452f799bfe840676ee341124611/Strongyloidiasis.webp.png)
![Prevalence Strongyloides stercoralis[51]](./_assets_/0c70a452f799bfe840676ee341124611/Epidemiology_Strongyloides_stercoralis.png)