Remibrutinib
 | |
| Clinical data | |
|---|---|
| Trade names | Rhapsido |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
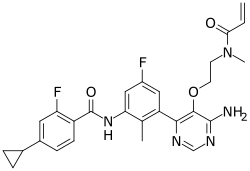
| Formula | C27H27F2N5O3 |
| Molar mass | 507.542 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Remibrutinib, sold under the brand name Rhapsido, is a medication used for the treatment of chronic spontaneous urticaria.[1] Remibrutinib is an oral, small molecule kinase inhibitor that inhibits Bruton's tyrosine kinase (BTK).[1] It is taken by mouth.[1]
Medical uses
Remibrutinib is indicated for the treatment of chronic spontaneous urticaria in adults who remain symptomatic despite H1 antihistamine treatment[1]
Side effects
Remibrutinib is generally well-tolerated in patients with chronic spontaneous urticaria, but common side effects include upper respiratory tract infections, headache, nasopharyngitis, and mild gastrointestinal symptoms such as diarrhea and nausea. Most adverse events are classified as mild to moderate, with serious side effects being rare; elevations in liver enzymes and transient neutropenia have also been reported but typically do not require treatment discontinuation.[2][3]
Mechanism of action
Remibrutinib is a selective inhibitor of Bruton's tyrosine kinase (BTK), a key signaling protein found in mast cells and basophils that regulates their activation and degranulation via pathways involving the FcεRI and B-cell receptor. By irreversibly binding to BTK, remibrutinib blocks intracellular signaling required for the release of histamine and other inflammatory mediators, thereby reducing the immune cell-driven processes underlying chronic spontaneous urticaria. This mechanism enables suppression of urticaria symptoms upstream from antihistamines, and may also modulate autoimmune activity in some patients.[3]
Society and culture
Legal status
Remibrutinib was approved for medical use in the United States in September 2025.[4]
Names
Remibrutinib is the international nonproprietary name.[5]
Remibrutinib is sold under the brand name Rhapsido.[4]
References
- 1 2 3 4 5 "RHAPSIDO (remibrutinib) tablets, for oral use" (PDF). Novartis.
Highlights of Prescribing Information
- ↑ Burhan M, Ashraf S, Ali A, Shahid I, Ahmed J, Shah MS, et al. (September 2025). "Safety and Efficacy of Remibrutinib for Chronic Spontaneous Urticaria: A Systematic Review and Meta-Analysis". International Archives of Allergy and Immunology: 1–12. doi:10.1159/000548302. PMID 40911497.
- 1 2 Bożek A, Reich A (May 2025). "Evaluating remibrutinib in the treatment of chronic spontaneous urticaria". Immunotherapy. 17 (7): 479–484. doi:10.1080/1750743X.2025.2510892. PMID 40455080.
- 1 2 "Novartis receives FDA approval for Rhapsido (remibrutinib), the only oral, targeted BTKi treatment for chronic spontaneous urticaria (CSU)" (Press release). Novartis Pharmaceuticals. 30 September 2025. Retrieved 1 October 2025 – via PR Newswire.
- ↑ World Health Organization (2020). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 83". WHO Drug Information. 34 (1). hdl:10665/339768.
Further reading
- Maurer, Marcus; Berger, William; Giménez-Arnau, Ana; Hayama, Koremasa; Jain, Vipul; Reich, Adam; et al. (December 2022). "Remibrutinib, a novel BTK inhibitor, demonstrates promising efficacy and safety in chronic spontaneous urticaria". The Journal of Allergy and Clinical Immunology. 150 (6): 1498–1506.e2. doi:10.1016/j.jaci.2022.08.027. hdl:10230/55511. ISSN 1097-6825. PMID 36096203.
- Maurer, Marcus; Giménez-Arnau, Ana; Jain, Vipul; Tillinghast, Jeffrey; Tolcachier, Alberto; Nigen, Simon; et al. (February 2022). "Remibrutinib Treatment Improves Quality of Life in Patients with Chronic Spontaneous Urticaria". Journal of Allergy and Clinical Immunology. 149 (2): AB179. doi:10.1016/j.jaci.2021.12.589. S2CID 246522006.
External links
- Clinical trial number NCT05030311 for "A Phase 3 Study of Efficacy and Safety of Remibrutinib in the Treatment of CSU in Adults Inadequately Controlled by H1 Antihistamines (REMIX-1)" at ClinicalTrials.gov
- Clinical trial number NCT05032157 for "A Phase 3 Study of Efficacy and Safety of Remibrutinib in the Treatment of CSU in Adults Inadequately Controlled by H1-antihistamines (REMIX-2)" at ClinicalTrials.gov