Spesolimab
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized |
| Target | Interleukin 36 receptor (IL1RL2/IL1RAP) |
| Names | |
| Trade names | Spevigo |
| Other names | BI-655130, spesolimab-sbzo |
| Clinical data | |
| Main uses | Generalized pustular psoriasis (GPP)[1] |
| Side effects | Tiredness, nausea, headache, itchiness, bruising at the site of administration, urinary tract infection[1] |
| Routes of use | Intravenous |
| Typical dose | 900 mg[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C6480H9988N1736O2012S46 |
| Molar mass | 145880.08 g·mol−1 |
Spesolimab, sold under the brand name Spevigo, is a medication used to treat a flair of generalized pustular psoriasis (GPP).[1] It is given by gradual injection into a vein.[1]
Common side effects include tiredness, nausea, headache, itchiness, bruising at the site of administration, and urinary tract infection.[1] Other side effects may include infection, allergic reactions, and infusion reactions.[1] It is a monoclonal antibody that blocks interleukin-36 receptor (IL1RL2/IL1RAP).[1][2]
Spesolimab was approved for medical use in the United States in 2022.[1] It is recommended for approval in Europe but is not in the approval process in the United Kingdom as of 2022.[3] In the United States it costs about 54,000 USD per dose as of 2022.[4]
Medical uses
Dosage
It is generally given as a dose of 900 mg, which may be repeated after 1 week.[1]
Mechanism of action
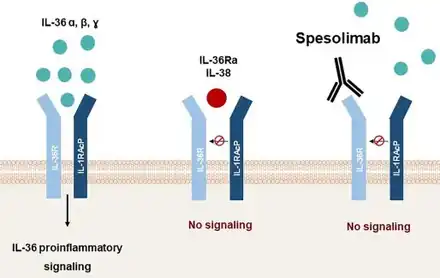
In terms of the mode of action we find that spesolimab binds to the IL-36 receptor , blocking its activation by IL-36 cytokines and thereby inhibiting downstream fibrotic signaling pathways. By targeting IL-36R, it modulates the immune response, reducing the neutrophilic inflammation responsible for the painful pustular eruptions.However the precise mechanism connecting IL-36R inhibition to symptom relief remains unclear[5]
Society and culture
Legal status
On 13 October 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a conditional marketing authorization for the medicinal product Spevigo, intended for the treatment of flares in adult patients with generalised pustular psoriasis.[6]
The applicant for this medicinal product is Boehringer Ingelheim International GmbH.[6]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 "Spevigo- spesolimab-sbzo injection". DailyMed. 1 September 2022. Archived from the original on 16 October 2022. Retrieved 16 October 2022.
- ↑ Ratnarajah K, Jfri A, Litvinov IV, Netchiporouk E (2020). "Spesolimab: A Novel Treatment for Pustular Psoriasis". Journal of Cutaneous Medicine and Surgery. 24 (2): 199–200. doi:10.1177/1203475419888862. PMID 32208020. S2CID 214641620.
- ↑ "Spesolimab". SPS - Specialist Pharmacy Service. 3 May 2019. Archived from the original on 17 May 2022. Retrieved 13 December 2022.
- ↑ "Spevigo Prices, Coupons, Copay & Patient Assistance". Drugs.com. Archived from the original on 20 December 2023. Retrieved 13 December 2022.
- ↑ 5.0 5.1 Bernardo, Diana; Thaçi, Diamant; Torres, Tiago (1 January 2024). "Spesolimab for the Treatment of Generalized Pustular Psoriasis". Drugs. 84 (1): 45–58. doi:10.1007/s40265-023-01988-0. ISSN 1179-1950. Archived from the original on 12 August 2024. Retrieved 20 August 2025.
- ↑ 6.0 6.1 "Spevigo: Pending EC decision". European Medicines Agency. 14 October 2022. Archived from the original on 15 October 2022. Retrieved 15 October 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
External links
| External sites: |
|
|---|---|
| Identifiers: |