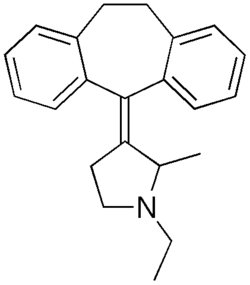
Piroheptine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H25N |
| Molar mass | 303.449 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Piroheptine (brand name Trimol) is an anticholinergic and antihistamine used as an antiparkinsonian agent.
Piroheptine was observed to prevent the reuptake of dopamine and is therefore a DRI.[1][2]
Piroheptine comes from a family of drugs that includes pridefine and etifelmine.
Synthesis
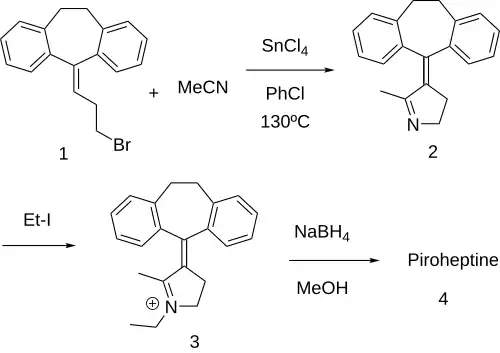
Piroheptine can be synthesized starting from 5-(3-bromopropylidene)-10,11-dihydro-5H-dibenzo[a,d]cycloheptene (1) and acetonitrile which react via catalysis by stannic chloride (SnCl4) to give 2-methyl-3-(10,11-dihydro-5H-dibenzo[a,d] cycloheptene-5-ylidene)-1-pyrroline (2). Quaternization of the product with ethyl iodide affords the alkyl immonium ion (3). Reduction of the Schiff base with sodium borohydride then affords the product, piroheptine (4).[3][4]
References
- ↑ Saitoh T (February 1988). "Suppression of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity in mouse brain by piroheptine and trihexyphenidyl". Journal of the Neurological Sciences. 83 (2–3): 161–166. doi:10.1016/0022-510X(88)90065-2. PMID 3258627. S2CID 25230405.
- ↑ Ohashi T, Akita H, Tamura T, Noda K, Honda F (June 1972). "Effect of piroheptine, a new antiparkinson drug, on dopamine uptake into synaptosomes from corpus striatum of rat brain". Arzneimittel-Forschung. 22 (6): 966–972. PMID 5068358.
- ↑ Yoshio Deguchi, Naomichi Kato, Hiroshi Nojima, U.S. patent 3,454,595 (1969 to Fujisawa Pharmaceutical Co).
- ↑ Umio, S., Hitomi, M., Nojima, H., Kumadaki, N., Ueda, I., Kanaya, T., Deguchi, Y. (September 1972). "Synthesis and pharmacological properties of 3-(10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-1,2-dialkylpyrrolidine derivatives". Journal of Medicinal Chemistry. 15 (9): 891–894. doi:10.1021/jm00279a004. PMID 4403249.