Vaniprevir
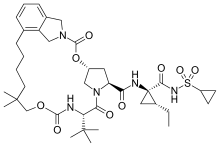 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.207.830 |
| Chemical and physical data | |
| Formula | C38H55N5O9S |
| Molar mass | 757.94 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Vaniprevir (MK-7009) is a macrocyclic hepatitis C virus (HCV) NS3/4A protease inhibitor, developed by Merck & Co., which is currently in clinical testing.[1] In Japan, it was approved for treating hepatitis C in 2014 under the brand name Vanihep.[2][3]
References
- ↑ McCauley JA, McIntyre CJ, Rudd MT, Nguyen KT, Romano JJ, Butcher JW, et al. (March 2010). "Discovery of vaniprevir (MK-7009), a macrocyclic hepatitis C virus NS3/4a protease inhibitor". J. Med. Chem. 53 (6): 2443–63. doi:10.1021/jm9015526. PMID 20163176.
- ↑ "First recommendation for HCV drug vaniprevir, in Japan". datamonitorhealthcare.com. September 25, 2014.
- ↑ "New Drugs Approved" (PDF). Pharmaceuticals and Medical Devices Agency. Archived from the original (PDF) on 2015-05-13. Retrieved 2015-08-22.