Coblopasvir
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
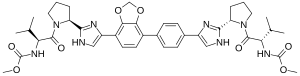
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C41H50N8O8 |
| Molar mass | 782.899 g·mol−1 |
InChI
| |
Coblopasvir is a pharmaceutical drug for the treatment of hepatitis C.[1] It is a pan-genotypic inhibitor of HCV nonstructural protein 5A.[2]
In China, it is approved for use in combination with sofosbuvir for treating naïve or interferon‐experienced adults chronically monoinfected patients with HCV of genotype 1, 2, 3 and 6, with or without compensated cirrhosis.[2]
References
- ↑ Rao H, Song G, Li G, Yang Y, Wu X, Guan Y, et al. (January 2020). "Safety and efficacy of coblopasvir and sofosbuvir in patients with genotypes 1, 2, 3 and 6 HCV infections without or with compensated cirrhosis". Journal of Viral Hepatitis. 27 (1): 45–51. doi:10.1111/jvh.13208. PMID 31520460.
- 1 2 Gao Y, Kong F, Li G, Li C, Zheng S, Lin J, et al. (November 2020). "Coblopasvir and sofosbuvir for treatment of chronic hepatitis C virus infection in China: A single-arm, open-label, phase 3 trial". Liver International. 40 (11): 2685–2693. doi:10.1111/liv.14633. PMC 7702130. PMID 33047868.