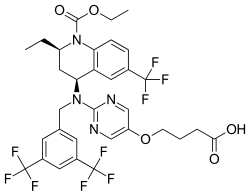
Obicetrapib
 | |
| Clinical data | |
|---|---|
| Other names | TA-8995; AMG-899 |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C32H31F9N4O5 |
| Molar mass | 722.609 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Obicetrapib is an experimental CETP inhibitor that is intended to treat dyslipidemia. In a clinical trial, as an add-on to statins, compared with placebo, it decreased concentrations of LDL-C (by up to 51%), apolipoprotein B (by up to 30%) and non-high-density lipoprotein cholesterol (non-HDL-C) (by up to 44%), and increased HDL-C concentration (by up to 165%).[1][2] As of 2023, it is in a Phase III trial.[3]
History
Obicetrapib was initially developed by Amgen as AMG-899 and was abandoned in 2017.[4] In 2020, Amgen licensed the drug to NewAmsterdam Pharma.[5] In 2022, NewAmsterdam Pharma licensed the drug to Menarini Group.[6]
References
- ↑ Ballantyne, Christie M.; Ditmarsch, Marc; Kastelein, John JP; Nelson, Adam J.; Kling, Douglas; Hsieh, Andrew; Curcio, Danielle L.; Maki, Kevin C.; Davidson, Michael H.; Nicholls, Stephen J. (July 2023). "Obicetrapib plus ezetimibe as an adjunct to high-intensity statin therapy: A randomized phase 2 trial". Journal of Clinical Lipidology. 17 (4): 491–503. doi:10.1016/j.jacl.2023.05.098. PMID 37277261.
- ↑ Nicholls, Stephen J.; Ditmarsch, Marc; Kastelein, John J.; Rigby, Scott P.; Kling, Douglas; Curcio, Danielle L.; Alp, Nicholas John; Davidson, Michael H. (August 2022). "Lipid lowering effects of the CETP inhibitor obicetrapib in combination with high-intensity statins: a randomized phase 2 trial". Nature Medicine. 28 (8): 1672–1678. doi:10.1038/s41591-022-01936-7. ISSN 1546-170X. PMID 35953719.
- ↑ "NewAmsterdam Pharma concludes enrolment in LDL lowering therapy trial". Clinical Trials Arena. 26 July 2023.
- ↑ Amgen kills off CETP inhibitor after seeing Merck data
- ↑ NewAmsterdam bags $196M to resurrect Amgen's discarded CETP drug
- ↑ "Menarini Group's Obicetrapib and Obicetrapib/Ezetimibe Marketing Authorisation Applications Accepted for Review by the European Medicines Agency (EMA) for the Treatment of adults with primary hypercholesterolaemia (Heterozygous familial and non-familial)".