Govorestat
 | |
| Clinical data | |
|---|---|
| Other names | AT-007 |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
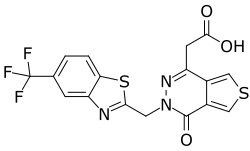
| Formula | C17H10F3N3O3S2 |
| Molar mass | 425.40 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Govorestat (AT-007) is an aldose reductase inhibitor and experimental drug to treat galactosemia[1] and sorbitol dehydrogenase deficiency.[2]
After a report circulating on the internet accused the developer Applied Therapeutics of cutting corners in its studies of the drug, the FDA put a hold on it in 2020. Applied Therapeutics said that the report was a fraudulent attempt to manipulate its stock price.[3]
In February 2024, the FDA granted priority review states to an application from Applied Therapeutics to use govorestat to treat galactosemia, a metabolic disease.[4] The FDA then rejected the company’s application in November 2024, and issued a warning letter to then-President Shoshana Shendelman informing her of “objectionable conditions” observed during an FDA inspection earlier that year.[5]
Society and culture
Legal status
In December 2024, Advanz Pharma Limited withdrew its application for a marketing authorization of Nugalviq for the treatment of classic galactosaemia, a condition where the body cannot break down a sugar called galactose.[6]
References
- ↑ Bailey, Evan; Wang, Stella; Saltonstall, Laura; Perfetti, Riccardo; Shendelman, Shoshana (March 2022). "OP005: AT-007 significantly reduces toxic galactitol in ACTION-galactosemia kids - the 1st therapeutic interventional clinical trial in children with classic galactosemia". Genetics in Medicine. 24 (3): S336. doi:10.1016/j.gim.2022.01.556. S2CID 247603696.
- ↑ "CTG Labs - NCBI". clinicaltrials.gov. 22 September 2023. Retrieved 5 November 2023.
- ↑ "Bad to worse for Applied Therapeutics as FDA slaps partial clinical hold on rare drug". Retrieved 4 November 2023.
- ↑ "Applied Therapeutics Announces FDA Acceptance and Priority Review of New Drug Application for Govorestat for the Treatment of Classic Galactosemia". Applied Therapeutics. 28 February 2024.
- ↑ Research, Center for Drug Evaluation and (3 December 2024). "Applied Therapeutics, Inc. - 696833 - 12/03/2024". FDA. Retrieved 8 July 2025.
- ↑ "Nugalviq EPAR". European Medicines Agency (EMA). 10 December 2024. Retrieved 16 February 2025.