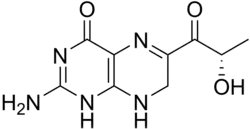
Sepiapterin
 | |
| Clinical data | |
|---|---|
| Trade names | Sephience |
| Other names | Sephience |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a625095 |
| License data |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C9H11N5O3 |
| Molar mass | 237.219 g·mol−1 |
SMILES
| |
InChI
| |
Sepiapterin, sold under the brand name Sephience, is a medication used for the treatment of hyperphenylalaninemia.[4][5] Sepiapterin is a phenylalanine hydroxylase activator.[2] It is also metabolite that is naturally synthesized in the human body.
The most common side effects are upper respiratory tract infection, headache, diarrhea, abdominal pain, hyperphenylalaninemia and discoloration of feces.[4]
Biochemistry
Sepiapterin is a naturally produced metabolite that can in turn be metabolized into tetrahydrobiopterin via a salvage pathway.[6] Tetrahydrobiopterin is an essential cofactor in humans for breakdown of phenylalanine and a catalyst of the metabolism of phenylalanine, tyrosine, and tryptophan to precursors of the neurotransmitters dopamine and serotonin.[7]
Medical uses
Sepiapterin is indicated for the treatment of hyperphenylalaninemia in people with phenylketonuria.[2][4]
Side effects
The most common side effects are upper respiratory tract infection, headache, diarrhea, abdominal pain, hyperphenylalaninemia and discoloration of feces.[4]
Mechanism of action
Sepiapterin is a metabolite that is naturally synthesized in the human body. Synthetic sepiapterin also functions as a prodrug that serves as a precursor to tetrahydrobiopterin (BH4). The therapeutic effect of sepiapterin results from its conversion to BH4, which functions as a cofactor for phenylalanine hydroxylase (PAH). This enzymatic activity enables patients with phenylketonuria (PKU) to better metabolize phenylalanine, thereby reducing its neurotoxic concentration in the blood.[8]
Society and culture
Legal status
In April 2025, the Committee for Medicinal Products for Human Use of the European Medicines Agency adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Sephience, intended for the treatment of hyperphenylalaninemia in adults and children with phenylketonuria.[4] The applicant for this medicinal product is PTC Therapeutics International Limited.[4] Sepiapterin was authorized for medical use in the European Union in June 2025.[4][5]
Sepiapterin was approved for medical use in the United States in July 2025.[2]
Research
Deficiency of tetrahydrobiopterin can cause toxic buildup of phenylalanine (phenylketonuria) as well as deficiencies of dopamine, norepinephrine, and epinephrine, leading to dystonia and other neurological illnesses. This has led to clinical study of sepiapterin in humans to treat tetrahydrobiopterin deficiency.[9]
Since atherosclerosis and other circulatory diseases associated with diabetes are also associated with tetrahydrobiopterin deficiency, animal studies of the value of sepiaterin in these vascular diseases have been done. These studies show that relaxation of the blood vessels studied was impaired after animals were given sepiapterin, even though their levels of tetrahydrobiopterin were replenished.[10]
References
- ↑ "Sephience (Ptc Therapeutics Australia Pty Limited)". Therapeutic Goods Administration (TGA). 24 September 2025. Retrieved 20 October 2025.
- 1 2 3 4 "Sephience- sepiapterin powder". DailyMed. 28 July 2025. Retrieved 20 October 2025.
- ↑ "Sephience- sepiapterin powder". DailyMed. 2 October 2025. Retrieved 20 October 2025.
- 1 2 3 4 5 6 7 8 "Sephience EPAR". European Medicines Agency (EMA). 25 April 2025. Retrieved 2 May 2025. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- 1 2 3 "Sephience Product information". Union Register of medicinal products. 25 June 2025. Retrieved 27 June 2025.
- ↑ Hasegawa H, Sawabe K, Nakanishi N, Wakasugi OK (December 2005). "Delivery of exogenous tetrahydrobiopterin (BH4) to cells of target organs: role of salvage pathway and uptake of its precursor in effective elevation of tissue BH4". Molecular Genetics and Metabolism. 86 Suppl 1: S2–10. doi:10.1016/j.ymgme.2005.09.002. PMID 16256391.
- ↑ Werner ER, Blau N, Thöny B (September 2011). "Tetrahydrobiopterin: biochemistry and pathophysiology". The Biochemical Journal. 438 (3): 397–414. doi:10.1042/BJ20110293. PMID 21867484.
- ↑ "Sepiapterin". DrugBank.
- ↑ Smith N, Longo N, Levert K, Hyland K, Blau N (April 2019). "Phase I clinical evaluation of CNSA-001 (sepiapterin), a novel pharmacological treatment for phenylketonuria and tetrahydrobiopterin deficiencies, in healthy volunteers". Molecular Genetics and Metabolism. 126 (4): 406–412. doi:10.1016/j.ymgme.2019.02.001. PMID 30922814. S2CID 85564348.
- ↑ Vásquez-Vivar J, Duquaine D, Whitsett J, Kalyanaraman B, Rajagopalan S (October 2002). "Altered tetrahydrobiopterin metabolism in atherosclerosis: implications for use of oxidized tetrahydrobiopterin analogues and thiol antioxidants". Arteriosclerosis, Thrombosis, and Vascular Biology. 22 (10): 1655–1661. doi:10.1161/01.ATV.0000029122.79665.D9. PMID 12377745.