Zatolmilast
 | |
| Clinical data | |
|---|---|
| Other names | BPN-14770 |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
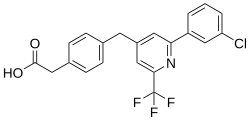
| Formula | C21H15ClF3NO2 |
| Molar mass | 405.80 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Zatolmilast is a investigational new drug that is being evaluated to treat fragile X syndrome.[1] It is a PDE4D allosteric inhibitor.[2]
It works by inhibiting breakdown of cyclic AMP (cAMP), with increased cAMP levels thought to increase neuronal connectivity. It has shown increased caregiver symptoms ratings and cognitive scores in a small clinical trial of 30 adults with fragile X syndrome.[3]
References
- ↑ "Zatolmilast - Tetra Therapeutics". AdisInsight. Springer Nature Switzerland AG.
- ↑ Blauvelt A, Langley RG, Gordon KB, Silverberg JI, Eyerich K, Sommer MO, et al. (December 2023). "Next Generation PDE4 Inhibitors that Selectively Target PDE4B/D Subtypes: A Narrative Review". Dermatology and Therapy. 13 (12): 3031–3042. doi:10.1007/s13555-023-01054-3. PMC 10689637. PMID 37924462.
- ↑ Hagerman RJ, Hagerman PJ (17 July 2025). "The Spectrum of Fragile X Disorders". New England Journal of Medicine. 393 (3): 281–288. doi:10.1056/NEJMra2300487. PMID 40673587.