Zanidatamab
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized (from mouse) |
| Target | HER2 |
| Names | |
| Trade names | Ziihera |
| Other names | ZW25, zanidatamab-hrii |
| Clinical data | |
| Drug class | Antineoplastic |
| Routes of use | Intravenous infusion |
| Legal | |
| License data |
|
| Legal status | |
| Identifiers | |
| CAS Number |
|
| DrugBank | |
| UNII | |
| KEGG | |
| ATC code | |
| Chemical and physical data | |
| Formula | C5553H8526N1482O1726S36 |
| Molar mass | 124818.10 g·mol−1 |
Zanidatamab, sold under the brand name Ziihera, is a humanized monoclonal antibody used for the treatment of HER2-positive biliary tract cancer.[1][2] It is an IgG-like bispecific HER2-directed antibody directed against two non-overlapping domains of HER2.[1][2][3] Zanidatamab is produced in Chinese hamster ovary cells.[1]
The most common adverse reactions include diarrhea, infusion-related reaction, abdominal pain, and fatigue.[2]
Zanidatamab was approved for medical use in the United States in November 2024.[2][4] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication.[5]
Medical uses
Zanidatamabis indicated for the treatment of adults with previously treated, unresectable or metastatic HER2-positive (IHC 3+) biliary tract cancer, as detected by an FDA-approved test.[1][2]
Adverse effects
The US Food and Drug Administration prescribing information contains a boxed warning for embryo-fetal toxicity.[2]
The most common adverse reactions include diarrhea, infusion-related reactions, abdominal pain, and fatigue.[2]
Mechanism of action
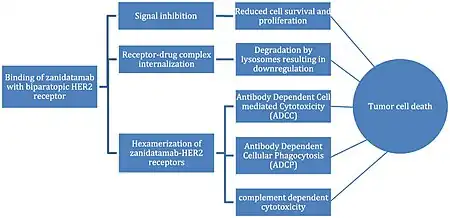
In terms of the mode of action we find that zanidatamab binds to two non-overlapping epitopes on HER2 receptor. Zanidatamab reduces HER2 levels on cell surface and disrupts downstream signaling pathways(pHER3 and pAKT)[6][7]
Zanidatamab recruits immune cells(NK cells) to kill HER2-expressing tumor cells, it facilitates engulfment of tumor cells by macrophages and it activates the complement system to lyse tumor cells[6]
History
Efficacy was evaluated in HERIZON-BTC-01 (NCT04466891), an open-label multicenter, single-arm trial in 62 participants with unresectable or metastatic HER2-positive (IHC3+) biliary tract cancer.[2] Participants were required to have received at least one prior gemcitabine-containing regimen in the advanced disease setting.[2]
The US Food and Drug Administration (FDA) granted the application for zanidatamab priority review, breakthrough therapy, and orphan drug designations.[2]
Society and culture
Legal status
Zanidatamab was approved for medical use in the United States in November 2024.[2][8][9]
In April 2025, the Committee for Medicinal Products for Human Use of the European Medicines Agency adopted a positive opinion, recommending the granting of a conditional marketing authorization for the medicinal product Ziihera, intended for the treatment of adults with unresectable locally advanced or metastatic HER2-positive biliary tract cancer.[10] The applicant for this medicinal product is Jazz Pharmaceuticals Ireland Limited.[10]
Names
Zanidatamab is the international nonproprietary name.[11]
Zanidatamab is sold under the brand name Ziihera.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Ziihera- zanidatamab-hrii injection, powder, lyophilized, for solution". DailyMed. 20 November 2024. Archived from the original on 30 November 2024. Retrieved 28 November 2024.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 "FDA grants accelerated approval to zanidatamab-hrii for previously treated unresectable or metastatic HER2-positive biliary tract cancer". U.S. Food and Drug Administration (FDA). 21 November 2024. Archived from the original on 21 November 2024. Retrieved 23 November 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Bhagyalalitha M, Handattu Shankaranarayana A, Arun Kumar S, Singh M, Pujar KG, Bidye D, et al. (October 2024). "Advances in HER2-Targeted Therapies: From monoclonal antibodies to dual inhibitors developments in cancer treatment". Bioorganic Chemistry. 151: 107695. doi:10.1016/j.bioorg.2024.107695. PMID 39137598.
- ↑ "Cancer Accelerated Approvals". U.S. Food and Drug Administration (FDA). 1 October 2024. Archived from the original on 27 October 2021. Retrieved 6 December 2024.
- ↑ New Drug Therapy Approvals 2024. U.S. Food and Drug Administration (FDA) (Report). January 2025. Archived from the original (PDF) on 21 January 2025. Retrieved 21 January 2025.
- ↑ 6.0 6.1 6.2 Kanwal W, Narjis K, Musani S, Nancy F, Qureshi L, Mudasir M, et al. (1 March 2025). "Exploring Zanidatamab's efficacy across HER2-positive Malignancies: a narrative review". BMC cancer. 25 (1): 382. doi:10.1186/s12885-025-13749-1. ISSN 1471-2407.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ↑ "Zanidatamab-hrii - NCI". www.cancer.gov. 23 December 2024. Archived from the original on 20 June 2025. Retrieved 6 July 2025.
- ↑ "Novel Drug Approvals for 2024". U.S. Food and Drug Administration (FDA). 1 October 2024. Archived from the original on 19 April 2024. Retrieved 29 November 2024.
- ↑ "Jazz Pharmaceuticals Announces U.S. FDA Approval of Ziihera (zanidatamab-hrii) for the Treatment of Adults with Previously Treated, Unresectable or Metastatic HER2-positive (IHC 3+) Biliary Tract Cancer (BTC)" (Press release). Jazz Pharmaceuticals. 20 November 2024. Archived from the original on 23 November 2024. Retrieved 23 November 2024 – via PR Newswire.
- ↑ 10.0 10.1 "Ziihera EPAR". European Medicines Agency (EMA). 25 April 2025. Retrieved 2 May 2025. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ World Health Organization (2020). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 83". WHO Drug Information. 34 (1). hdl:10665/339768.
External links
- "Zanidatamab-hrii". NCI Drug Dictionary. Archived from the original on 6 April 2025. Retrieved 29 May 2025.
- "Zanidatamab (Code C130010)". NCI Thesaurus. Archived from the original on 16 April 2025. Retrieved 29 May 2025.
- Clinical trial number NCT04466891 for "A Study of ZW25 (Zanidatamab) in Subjects With Advanced or Metastatic HER2-Amplified Biliary Tract Cancers (HERIZON-BTC-01)" at ClinicalTrials.gov