Dacomitinib
 | |
| Names | |
|---|---|
| Pronunciation | dak" oh mi' ti nib |
| Trade names | Vizimpro |
| Other names | PF-00299804 |
IUPAC name
| |
| Clinical data | |
| Drug class | EGFR inhibitor[1] |
| Main uses | Non-small-cell lung carcinoma (NSCLC)[2] |
| Routes of use | By mouth |
| Typical dose | 45 mg OD[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618055 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 80% |
| Protein binding | 98% |
| Metabolism | CYP2D6, CYP3A4 |
| Metabolites | O-desmethyl-dacomitinib |
| Elimination half-life | 70 hrs |
| Excretion | 79% faeces, 3% urine |
| Chemical and physical data | |
| Formula | C24H25ClFN5O2 |
| Molar mass | 469.95 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Dacomitinib, sold under the brand name Vizimpro, is a medication used to treat non-small-cell lung carcinoma (NSCLC).[2] Specifically it is used for cases with certain epidermal growth factor receptor (EGFR) mutations.[2] It is taken by mouth.[2]
Common side effects include diarrhea, rash, mouth inflammation, conjunctivitis, itching, liver problems, and nausea.[3] Other side effects may include interstitial lung disease.[3] Use in pregnancy may harm the baby.[2] It is a tyrosine kinase inhibitor of EGFR.[1]
Dacomitinib was approved for medical use in the United States in 2018 and Europe in 2019.[2][3] In the United States it costs about 14,300 USD per month as of 2021.[4] This amount in the United Kingdom costs the NHS about £2,700.[5]
Medical uses
Dosage
The typical dose is 45 mg per day.[3]
Mechanism of action
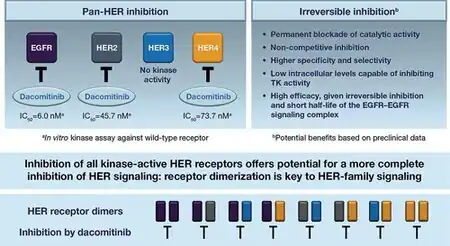
The mode of action of dacomitinib works by binding to kinase domains of EGFR family(EGFR/HER1, HER2, HER4). As a consequence this inhibits tyrosine kinase autophosphorylation, which causes a reduction in tumor growth[6]
Research
Dacomitinib has advanced to several Phase III clinical trials.The January 2014 results of the first trials were disappointing, with a failure to meet the study goals.[7][8][9]
Additional Phase III trials are ongoing.[7]
References
- ↑ 1.0 1.1 "Dacomitinib". NCI Drug Dictionary. National Cancer Institute, U.S. Department of Health and Human Services. Archived from the original on 28 April 2015. Retrieved 31 May 2021.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Dacomitinib Monograph for Professionals". Drugs.com. Archived from the original on 1 January 2022. Retrieved 17 December 2021.
- ↑ 3.0 3.1 3.2 3.3 3.4 "Vizimpro EPAR". European Medicines Agency (EMA). 5 June 2019. Archived from the original on 13 December 2019. Retrieved 13 December 2019.
- ↑ "Vizimpro Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 28 May 2019. Retrieved 17 December 2021.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1021. ISBN 978-0857114105.
- ↑ 6.0 6.1 Reungwetwattana, Thanyanan; Rohatgi, Nitesh; Mok, Tony S.; Prabhash, Kumar (4 May 2021). "Dacomitinib as first-line treatment for EGFR mutation-positive non-small cell lung cancer". Expert Review of Precision Medicine and Drug Development. 6 (3): 161–171. doi:10.1080/23808993.2021.1909420. Archived from the original on 30 August 2024. Retrieved 29 August 2024.
- ↑ 7.0 7.1 Chustecka Z (27 January 2014). "Dacomitinib Fails in Pretreated Non-small Cell Lung Cancer". Medscape. Archived from the original on 13 June 2017. Retrieved 31 May 2021.
- ↑ Taylor P (28 January 2014). "Blow to Pfizer as dacomitinib fails in lung cancer trials". pmlive.com. Archived from the original on 5 March 2019. Retrieved 31 May 2021.
- ↑ "Pfizer Announces Top-Line Results From Two Phase 3 Trials Of Dacomitinib In Patients With Refractory Advanced Non-Small Cell Lung Cancer". Pfizer Press Release. 27 January 2014. Archived from the original on 11 June 2019. Retrieved 31 May 2021.
External links
| External sites: |
|
|---|---|
| Identifiers: |