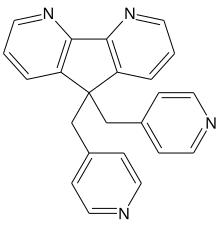
Sibopirdine
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | sus |
| Metabolism | sus |
| Excretion | sus |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H18N4 |
| Molar mass | 350.425 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Synthesis
Patent:[2]
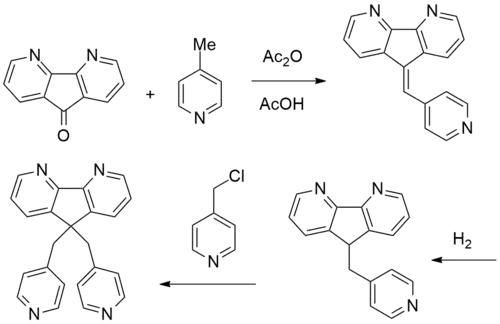
4,5-Diazafluoren-9-one [50890-67-0] (1) is condensed with 4 molar equivalents of 4-picoline [108-89-4] (2) in the presence of 2 equivalents of acetic anhydride and acetic acid. The intermediate from this step may originally be a tertiary alcohol which then dehydrates under the reaction conditions to give [150896-71-2] (3). The olefin can then be reduced either by catalytic hydrogenation over 5% Pd/C or by sodium borohydride in alcohol to give [150896-72-3] (4). Alkylation with 4-picolyl chloride [10445-91-7] (5) in the presence of sodium hydroxide completed the synthesis of sibopirdine (6).
See also
References
- ↑ Wong YN, Quon CY, Holm KA, Burcham DL, Frey NL, Huang SM, Lam GN (February 1996). "Pharmacokinetics and metabolism of EXP921, a novel cognitive enhancer, in rats". Drug Metabolism and Disposition. 24 (2): 172–9. doi:10.1016/S0090-9556(25)07294-0. PMID 8742228.
- ↑ James H. Jensen, Timothy D. Costello, Leon De Brabander, Jr., Matthew E. Voss, U.S. patent 5,272,269 (1993 to DuPont Merck Pharmaceutical Company).