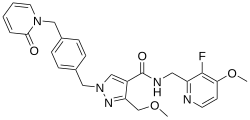
Sebetralstat
 | |
| Clinical data | |
|---|---|
| Trade names | Ekterly |
| Other names | KVD-900, KVD900 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a625087 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Plasma kallikrein inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C26H26FN5O4 |
| Molar mass | 491.523 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Sebetralstat, sold under the brand name Ekterly, is a medication used for the treatment of hereditary angioedema.[1] Sebetralstat is a plasma kallikrein inhibitor that suppresses the activation of the positive feedback mechanism of the kallikrein-kinin system, thereby reducing factor XIIa and additional plasma kallikrein production.[2] By inhibiting plasma kallikrein, sebetralstat reduces the production of bradykinin, thereby halting the progression of hereditary angioedema attacks.[2] It is taken by mouth.[1]
The most common side effect is headache.[4]
Sebetralstat was approved for medical use in the United States in July 2025,[4] and in the European Union in September 2025.[2][3]
Medical uses
Sebetralstat is indicated for the treatment of acute attacks of hereditary angioedema.[1][4]
Pharmacology
Sebetralstat is a plasma kallikrein inhibitor that contains the unusual 2-pyridone heterocycle.[5]
History
The US Food and Drug Administration (FDA) approved sebetralstat based on evidence from a clinical trial (KONFIDENT) that enrolled 110 participants with hereditary angioedema type I or II.[4] The trial was conducted at 66 sites in 20 countries, including Australia, Bulgaria, Canada, France, Germany, Greece, Hungary, Israel, Italy, Japan, Netherlands, New Zealand, North Macedonia, Poland, Portugal, Romania, Slovakia, Spain, the United Kingdom, and the United States.[4] In the study, participants tried three different treatments in random order: sebetralstat 600 mg, sebetralstat 300 mg, and a placebo (inactive pill that looks like the real medicine).[4] The main goal of the study was to measure how quickly sebetralstat started working.[4] Researchers tracked how long it took for people to feel at least "a little better" twice in a row within twelve hours of taking their first dose.[4]
Society and culture
Legal status
Sebetralstat was approved for medical use in the United States in July 2025.[6] The US Food and Drug Administration granted the application for sebetralstat orphan drug designation.[7]
In July 2025, the Committee for Medicinal Products for Human Use of the European Medicines Agency adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Ekterly, intended for the symptomatic treatment of acute attacks of hereditary angioedema in people aged twelve years of age and older.[2] The applicant for this medicinal product is KalVista Pharmaceuticals (Ireland) Ltd.[2] Hereditary angioedema is a rare, chronic, genetic, debilitating, and potentially life-threatening disorder characterized by recurrent and often unpredictable attacks of swelling in many parts of the body.[2] This is the first oral treatment for hereditary angioedema recommended for approval in the European Union.[2] Sebetralstat was authorized for medical use in the European Union in September 2025.[2][3]
Names
Sebetralstat is the international nonproprietary name.[8]
Sebetralstat is sold under the brand name Ekterly.[1]
References
- 1 2 3 4 5 "Ekterly- sebetralstat tablet". DailyMed. 7 July 2025. Retrieved 9 July 2025.
- 1 2 3 4 5 6 7 8 9 "Ekterly EPAR". European Medicines Agency (EMA). 25 July 2025. Retrieved 27 July 2025. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- 1 2 3 "Ekterly PI". Union Register of medicinal products. 18 September 2025. Retrieved 30 September 2025.
- 1 2 3 4 5 6 7 8 "Drug Trials Snapshots: Ekterly". U.S. Food and Drug Administration. 3 July 2025. Retrieved 30 September 2025.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Davie RL, Edwards HJ, Evans DM, Hodgson ST, Stocks MJ, Smith AJ, et al. (October 2022). "Sebetralstat (KVD900): A Potent and Selective Small Molecule Plasma Kallikrein Inhibitor Featuring a Novel P1 Group as a Potential Oral On-Demand Treatment for Hereditary Angioedema". Journal of Medicinal Chemistry. 65 (20): 13629–13644. doi:10.1021/acs.jmedchem.2c00921. PMC 9620001. PMID 36251573.
- ↑ "KalVista Pharmaceuticals Announces FDA Approval of Ekterly (sebetralstat), First and Only Oral On-demand Treatment for Hereditary Angioedema" (Press release). Kalvista. 7 July 2025. Retrieved 9 July 2025 – via Business Wire.
- ↑ "Sebetralstat Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). Retrieved 9 July 2025.
- ↑ World Health Organization (2022). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 87". WHO Drug Information. 36 (1). hdl:10665/352794.
External links
- "Sebetralstat (Code - C184930)". EVS Explore.
- Clinical trial number NCT05259917 for "A Phase III, Crossover Trial Evaluating the Efficacy and Safety of KVD900 (Sebetralstat) for On-Demand Treatment of Angioedema Attacks in Adolescent and Adult Patients With Hereditary Angioedema (HAE)" at ClinicalTrials.gov