Oveporexton
 | |
| Clinical data | |
|---|---|
| Other names | TAK-861; TAK861 |
| Routes of administration | Oral[1][2] |
| Drug class | Orexin OX2 receptor agonist; Wakefulness-promoting agent |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
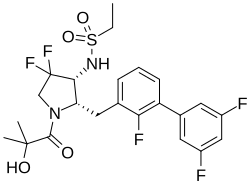
| Formula | C23H25F5N2O4S |
| Molar mass | 520.52 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Oveporexton (INN; developmental code name TAK-861) is an orexin receptor agonist and wakefulness-promoting agent which is under development for the treatment of narcolepsy (types 1 and 2) and idiopathic hypersomnia.[1][2][3] It is taken by mouth.[1][2]
The drug acts as a selective agonist of the orexin OX2 receptor.[1][2] It has wakefulness-promoting effects in animals, including in rodents and monkeys.[2] In addition, oveporexton has been found to be effective in the treatment of narcolepsy and cataplexy in phase 3 clinical trials in humans.[4][5][6] The drug is a first-in-class medication and targets the root symptomatic cause of narcolepsy (type 1) by remediating the orexin (hypocretin) deficiency that is present in the condition.[7][8][9]
Oveporexton is being developed by Takeda.[1] As of July 2025, it has completed phase 3 clinical trials for treatment of narcolepsy, whereas no recent development has been reported for treatment of idiopathic hypersomnia.[1][5][10] Takeda plans to submit a New Drug Application (NDA) of oveporexton for the treatment of narcolepsy to the United States Food and Drug Administration (FDA) in 2025.[5] Oveporexton is a follow-on and replacement compound for Takeda's earlier lead drug danavorexton (TAK-925), which is administered intravenously and stopped being developed due to unexpected liver toxicity findings.[10]
See also
- Orexin receptor § Agonists
- List of investigational narcolepsy and hypersomnia drugs
References
- 1 2 3 4 5 6 "Oveporexton". AdisInsight. 16 December 2024. Retrieved 26 February 2025.
- 1 2 3 4 5 Mitsukawa K, Terada M, Yamada R, Monjo T, Hiyoshi T, Nakakariya M, et al. (September 2024). "TAK-861, a potent, orally available orexin receptor 2-selective agonist, produces wakefulness in monkeys and improves narcolepsy-like phenotypes in mouse models". Scientific Reports. 14 (1) 20838. Bibcode:2024NatSR..1420838M. doi:10.1038/s41598-024-70594-1. PMC 11379823. PMID 39242684.
- ↑ Kallweit MS, Kallweit NP, Kallweit U (29 November 2023). "Pharmacological Treatments of Sleep–Wake Disorders: Update 2023". Clinical and Translational Neuroscience. 7 (4): 42. doi:10.3390/ctn7040042. ISSN 2514-183X.
- ↑ Walters J (7 October 2025). "Positive Data Presentation on Oveporexton for Narcolepsy". Psychiatric Times. Retrieved 7 October 2025.
- 1 2 3 Beaney A (14 July 2025). "Takeda's oral narcolepsy drug shines in two Phase III trials". Clinical Trials Arena. Retrieved 7 October 2025.
- ↑ Dauvilliers Y, Plazzi G, Mignot E, Lammers GJ, Del Río Villegas R, Khatami R, et al. (May 2025). "Oveporexton, an Oral Orexin Receptor 2-Selective Agonist, in Narcolepsy Type 1". The New England Journal of Medicine. 392 (19): 1905–1916. doi:10.1056/NEJMoa2405847. PMID 40367374.
{{cite journal}}: CS1 maint: overridden setting (link) - ↑ Abad VC (2023). "Pharmacological options for narcolepsy: are they the way forward?". Expert Rev Neurother. 23 (9): 819–834. doi:10.1080/14737175.2023.2249234. PMID 37585269.
- ↑ Matsuyama K (8 September 2025). "Takeda Nears First Therapy for Narcolepsy's Root Cause". Bloomberg.com. Archived from the original on 8 September 2025. Retrieved 7 October 2025.
- ↑ Vinluan F (9 September 2025). "Takeda Is Waking Up the Narcolepsy Market With First-in-Class Drug, But Alkermes Is on Its Heels". MedCity News. Retrieved 7 October 2025.
- 1 2 Mullard A (September 2025). "Leading orexin receptor agonist clears phase III for narcolepsy". Nat Rev Drug Discov. 24 (9): 655. doi:10.1038/d41573-025-00137-4. PMID 40775090.