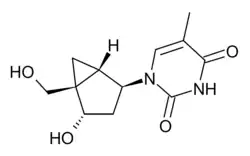
North-methanocarbathymidine
 | |
| Clinical data | |
|---|---|
| Trade names | N-MCT |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C12H16N2O4 |
| Molar mass | 252.270 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
North-Methanocarbathymidine (N-MCT) is an antiviral drug which is an analogue of thymidine, and shows activity against herpesviruses, orthopoxviruses and HIV,[1][2][3][4] though it has not been introduced into clinical use.
References
- ↑ Zhu W, Burnette A, Dorjsuren D, Roberts PE, Huleihel M, Shoemaker RH, et al. (December 2005). "Potent antiviral activity of north-methanocarbathymidine against Kaposi's sarcoma-associated herpesvirus". Antimicrobial Agents and Chemotherapy. 49 (12): 4965–73. doi:10.1128/AAC.49.12.4965-4973.2005. PMC 1315933. PMID 16304159.
- ↑ Marquez VE, Hughes SH, Sei S, Agbaria R (September 2006). "The history of N-methanocarbathymidine: the investigation of a conformational concept leads to the discovery of a potent and selective nucleoside antiviral agent". Antiviral Research. 71 (2–3): 268–75. doi:10.1016/j.antiviral.2006.04.012. PMID 16730077.
- ↑ Prichard MN, Kern ER (September 2010). "Antiviral Activity of 4'-thioIDU and Thymidine Analogs against Orthopoxviruses". Viruses. 2 (9): 1968–83. doi:10.3390/v2091968. PMC 3185742. PMID 21994716.
- ↑ De Clercq E (November 2013). "Highlights in antiviral drug research: antivirals at the horizon". Medicinal Research Reviews. 33 (6): 1215–48. doi:10.1002/med.21256. PMC 7168470. PMID 22553111.