LIT-001
 | |
| Clinical data | |
|---|---|
| Drug class | Oxytocin receptor agonist |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
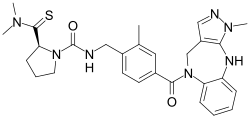
| Formula | C28H33N7O2S |
| Molar mass | 531.68 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
LIT-001 is a small-molecule oxytocin receptor agonist and vasopressin receptor mixed agonist and antagonist that was first described in the literature in 2018.[1][2][3][4] Along with TC OT 39 and WAY-267464, it is one of the first small-molecule oxytocin receptor agonists to have been developed.[1][2][4] LIT-001 has greatly improved pharmacokinetic properties relative to oxytocin, reduces social deficits in animal models, and may have potential use in the treatment of social disorders like autism in humans.[1][2][4] It is or was undergoing formal clinical development for such uses.[5][4]
Pharmacology
LIT-001 has greater selectivity for the oxytocin receptor over the vasopressin V1A receptor than the related compounds TC OT 39 and WAY-267464.[1][2] It shows antagonism of the V1A receptor only at high concentrations.[1][2] LIT-001 additionally acts as an agonist of the vasopressin V2 receptor, with this action occurring at similar concentrations as for the oxytocin receptor.[2][1] This is unlikely to influence the oxytocin receptor-related behavioral effects of LIT-001, as V2 receptors are not expressed in the brain.[1][2] However, it may influence fluid homeostasis, analogously to vasopressin.[1][2]
Given via peripheral administration, LIT-001 reduces social deficits in a mouse model of autism, specifically the μ-opioid receptor knockout mouse model.[1][2][3] It was the first small-molecule oxytocin receptor agonist to be shown to reduce social dysfunction in animals.[1][2] LIT-001 shows blood–brain barrier permeability and has a relatively long elimination half-life in rodents, giving it an advantageous drug profile relative to peptide oxytocin receptor agonists like oxytocin.[1][3][6][4] In the case of oxytocin, the amount estimated to enter the cerebrospinal fluid is only 0.002% with subcutaneous injection and at most 0.005% with intranasal administration, its half-life is only about 20 to 60 minutes, and it is not orally bioavailable, all of which greatly limit its potential usefulness as a central nervous system-acting medication.[1][2][4] These limitations of oxytocin may underlie limited effectiveness with oxytocin nasal spray in clinical trials.[1][2][4] Based on its positive social effects in animal models and its favorable pharmacokinetic properties, LIT-001 may have potential as a therapeutic agent in the treatment of social disorders in humans.[1][2][3]
The affinity (Ki) of LIT-001 for the human oxytocin receptor, where it acts as an agonist, is 226 nM, and its half maximal effective concentration (EC50) is 25 nM.[2] At the human vasopressin V1A receptor, where LIT-001 is an antagonist, its affinity (Ki) and half maximal inhibitory concentration (IC50) are 1253 nM and 5900 nM, respectively.[2] Finally, at the human vasopressin V2 receptor, where the drug functions as an agonist, its affinity (Ki) and EC50 are 1666 nM and 41 nM, respectively.[2] Based on the preceding EC50 and IC50 values, LIT-001 shows 236-fold selectivity for activating the oxytocin receptor over antagonizing the V1A receptor, whereas it has no appreciable selectivity for activating the oxytocin receptor over activating the V2 receptor (only 1.64-fold greater preference).[2]
Chemistry
LIT-001 is a small-molecule compound with the molecular formula C28H33N7O2S, a molecular weight of 531.7 g/mol, and a predicted log P of 1.95 to 2.8.[7][8] It is similar in structure to the earlier small-molecule oxytocin receptor agonists TC OT 39 and WAY-267,464.[1][2]
Development
LIT-001 was first described in the scientific literature in 2018.[3] It was developed by Marcel Hibert and colleagues at the Laboratory for Therapeutic Innovation (LIT) at the University of Strasbourg in France and by the Centre National de la Recherche Scientifique (French National Centre for Scientific Research).[5][4] Hibert had spent 20 years studying oxytocin before finally identifying LIT-001, a synthetic small-molecule oxytocin receptor agonist with favorable pharmacokinetic properties and effects.[4][3] The drug is being formally developed for potential treatment of autistic spectrum disorders.[5][4] It is in the preclinical research stage of development for this indication.[5][4] Hibert has disclosed that other more potent and selective small-molecule oxytocin receptor agonists have also since been discovered by his research group.[4] Formal development of these compounds is now being pursued.[4]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Nashar PE, Whitfield AA, Mikusek J, Reekie TA (2022). "The Current Status of Drug Discovery for the Oxytocin Receptor". Oxytocin. Methods Mol Biol. Vol. 2384. pp. 153–174. doi:10.1007/978-1-0716-1759-5_10. ISBN 978-1-0716-1758-8. PMID 34550574. S2CID 239090096.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Gulliver D, Werry E, Reekie TA, Katte TA, Jorgensen W, Kassiou M (January 2019). "Targeting the Oxytocin System: New Pharmacotherapeutic Approaches". Trends Pharmacol Sci. 40 (1): 22–37. doi:10.1016/j.tips.2018.11.001. hdl:1959.4/unsworks_81554. PMID 30509888. S2CID 54559394.
- 1 2 3 4 5 6 Frantz MC, Pellissier LP, Pflimlin E, Loison S, Gandía J, Marsol C, Durroux T, Mouillac B, Becker JA, Le Merrer J, Valencia C, Villa P, Bonnet D, Hibert M (October 2018). "LIT-001, the First Nonpeptide Oxytocin Receptor Agonist that Improves Social Interaction in a Mouse Model of Autism" (PDF). J Med Chem. 61 (19): 8670–8692. doi:10.1021/acs.jmedchem.8b00697. PMID 30199637. S2CID 52181935.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Boutaud, Anne-Sophie (9 January 2022). "Oxytocin, from love potion to medicine". CNRS News. Retrieved 15 October 2025.
After twenty years of research, we finally managed to overcome this obstacle and identified a synthetic molecule, called LIT-001, which acts in the same way as oxytocin. This was the first one that could restore social interaction in an animal model of autism5 following peripheral administration. Other, more potent and specific molecules have since been discovered and will soon be patented; at best, their preclinical and clinical development potential will require eight to ten years of industrial investment.
- 1 2 3 4 "Delving into the Latest Updates on LIT-001 with Synapse". Synapse. 2 August 2024. Retrieved 18 August 2024.
- ↑ Hilfiger L, Zhao Q, Kerspern D, Inquimbert P, Andry V, Goumon Y, Darbon P, Hibert M, Charlet A (February 2020). "A Nonpeptide Oxytocin Receptor Agonist for a Durable Relief of Inflammatory Pain". Sci Rep. 10 (1) 3017. Bibcode:2020NatSR..10.3017H. doi:10.1038/s41598-020-59929-w. PMC 7033278. PMID 32080303.
- ↑ "LIT-001 (free base)". PubChem. Retrieved 31 August 2024.
- ↑ "(2S)-2-(Dimethylcarbamothioyl)-N-{2-methyl-4-[(1-methyl-4,10-dihydropyrazolo[3,4-b][1,5]benzodiazepin-5(1H)-yl)carbonyl]benzyl}-1-pyrrolidinecarboxamide". ChemSpider. 2024-08-31. Retrieved 2024-08-31.