Fudosteine
 | |
| Clinical data | |
|---|---|
| Trade names | Cleanal |
| Other names | S-(3-Hydroxypropyl)-L-cysteine |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
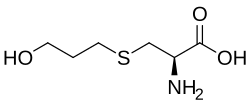
| Formula | C6H13NO3S |
| Molar mass | 179.23 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Fudosteine (Cleanal) is a mucolytic agent.[1] In Japan, it is approved for the treatment of bronchial asthma, chronic bronchitis, pulmonary emphysema, bronchiectasis, pulmonary tuberculosis, pneumoconiosis, atypical mycobacterial disease, and diffuse panbronchiolitis.[2]
Fudosteine works by increasing mucin secretion by inhibiting expression of the protein mucin 5AC.[3]
References
- ↑ Rhee CK, Kang CM, You MB, Yoon HK, Kim YK, Kim KH, et al. (November 2008). "Effect of fudosteine on mucin production". The European Respiratory Journal. 32 (5): 1195–1202. doi:10.1183/09031936.00018508. PMID 18579549.
- ↑ "Fudosteine". Inxight Drugs. National Center for Advancing Translational Sciences (NCATS), U.S. National Institutes of Health.
- ↑ Antonela Antoniu S (January 2009). "Fudosteine effects on mucin production". Expert Opinion on Investigational Drugs. 18 (1): 105–107. doi:10.1517/13543780802623863. PMID 19053887. S2CID 219294005.