Enmetazobactam
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
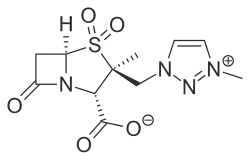
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C11H14N4O5S |
| Molar mass | 314.32 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Enmetazobactam (AAI-101) is an antibiotic adjuvant drug which acts as a beta-lactamase inhibitor, preventing the breakdown of other antibiotic drugs.[1] Enmetazobactam was invented by a team of scientists at Orchid Pharma in India and then out-licensed to Allecra Therapeutics for further development.[2] In the United States and European Union, enmetazobactam is approved for use in the combination cefepime/enmetazobactam (Exblifep).[3][4]
References
- ↑ Lanier C, Melton T, Covert K (September 2024). "Cefepime-Enmetazobactam: A Drug Review of a Novel Beta-Lactam/Beta-Lactamase Inhibitor". The Annals of Pharmacotherapy. 59 (6): 570–576. doi:10.1177/10600280241279904. PMID 39329253.
- ↑ US patent No.7687488B2 Novel 2-substituted methyl penam derivatives, https://worldwide.espacenet.com/patent/search/family/038949982/publication/US7687488B2?q=pn%3DUS7687488 Archived 5 April 2024 at the Wayback Machine
- ↑ New Drug Therapy Approvals 2024 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2025. Archived from the original on 21 January 2025. Retrieved 21 January 2025.
- ↑ "Exblifep EPAR". European Medicines Agency (EMA). 25 January 2024. Archived from the original on 4 February 2024. Retrieved 3 February 2024. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.