EG-2201
 | |
| Names | |
|---|---|
| IUPAC name
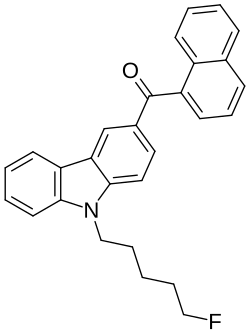
[9-(5-fluoropentyl)carbazol-3-yl]-naphthalen-1-ylmethanone | |
| Other names
(9-(5-fluoropentyl)-9H-carbazol-3-yl)(naphthalen-1-yl)methanone | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C28H24FNO |
| Molar mass | 409.504 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
EG-2201 (also known as NA-5F-PCZMO using EUDA systematic nomenclature[1]) is a synthetic cannabinoid derived from a carbazole core group.[2] It has been identified as a designer drug and is structurally related to other synthetic cannabinoids, such as EG-018 and MDMB-CHMCZCA. It is primarily used illicitly due to its psychoactive effects, which mimic delta-9-tetrahydrocannabinol (THC), the active ingredient in cannabis.[3]
Chemical properties
EG-2201 comprises a carbazole core group with a 5-fluoropentyl tail group, a methanone linker group, and a napthylenyl linked group. The use of a carbazole core group may increase CB2 receptor affinity compared to less bulky core groups like indoles and indazoles.[3][4]
Pharmacology
EG-2201 acts as an agonist of both human cannabinoid receptors, CB1 and CB2, at an affinity of 22.4 ± 12.8 nM and 4.36 ± 2.91 nM, respectively.[4]
Risks and toxicity
Limited toxicity studies exist for EG-2201, but related synthetic cannabinoids are associated with seizures, cardiovascular events, and psychiatric disturbances. Its metabolic byproducts may also be toxic.[5]
See also
References
- ↑ Pulver B, Fischmann S, Gallegos A, Christie R (March 2023). "EMCDDA framework and practical guidance for naming synthetic cannabinoids". Drug Testing and Analysis. 15 (3): 255–276. doi:10.1002/dta.3403. PMID 36346325. S2CID 253396419.
- ↑ Mogler, Lukas; Franz, Florian; Wilde, Maurice; Huppertz, Laura M.; Halter, Sebastian; Angerer, Verena; Moosmann, Bjoern; Auwärter, Volker (2018). "Phase I metabolism of the carbazole-derived synthetic cannabinoids EG-018, EG-2201, and MDMB-CHMCZCA and detection in human urine samples". Drug Testing and Analysis. 10 (9): 1417–1429. doi:10.1002/dta.2398. ISSN 1942-7611. PMID 29726116.
- 1 2 Potts, A. J.; Cano, C.; Thomas, S. H. L.; Hill, S. L. (2020-02-01). "Synthetic cannabinoid receptor agonists: classification and nomenclature". Clinical Toxicology. 58 (2): 82–98. doi:10.1080/15563650.2019.1661425. ISSN 1556-3650. PMID 31524007.
- 1 2 Schoeder, Clara T.; Hess, Cornelius; Madea, Burkhard; Meiler, Jens; Müller, Christa E. (16 April 2017). "Pharmacological evaluation of new constituents of "Spice": synthetic cannabinoids based on indole, indazole, benzimidazole and carbazole scaffolds". Forensic Toxicology. 36 (2): 385–403. doi:10.1007/s11419-018-0415-z. ISSN 1860-8965. PMC 6002460. PMID 29963207.
- ↑ Banister, Samuel D.; Connor, Mark (2018), Maurer, Hans H.; Brandt, Simon D. (eds.), "The Chemistry and Pharmacology of Synthetic Cannabinoid Receptor Agonist New Psychoactive Substances: Evolution", New Psychoactive Substances, vol. 252, Cham: Springer International Publishing, pp. 191–226, doi:10.1007/164_2018_144, ISBN 978-3-030-10560-0, PMID 30105473, retrieved 2025-01-02