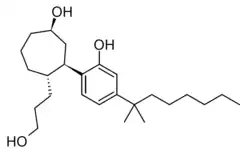
Cycloheptyl CP 55,940
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| Chemical and physical data | |
| Formula | C25H42O3 |
| Molar mass | 390.608 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Cycloheptyl CP 55,940 is a synthetic cannabinoid related to CP 55,940 but is a ring-expanded homologue with a cycloheptyl ring in place of the cyclohexyl ring. It was first synthesized by Pfizer in the 1980s.[1][2] It falls outside the definition of a "cyclohexylphenol derivative" since it does not have a cyclohexyl ring. Cycloheptyl CP 55,940 has similar potency to CP 55,940 itself, with an ED50 of 0.06 mg/kg in animal studies.[3]
See also
References
- ↑ Melvin LS, Milne GM, Johnson MR, Subramaniam B, Wilken GH, Howlett AC (November 1993). "Structure-activity relationships for cannabinoid receptor-binding and analgesic activity: studies of bicyclic cannabinoid analogs". Molecular Pharmacology. 44 (5): 1008–1015. doi:10.1016/S0026-895X(25)13256-2. PMID 8246904.
- ↑ Melvin LS, Milne GM, Johnson MR, Wilken GH, Howlett AC (November 1995). "Structure-activity relationships defining the ACD-tricyclic cannabinoids: cannabinoid receptor binding and analgesic activity". Drug Design and Discovery. 13 (2): 155–166. PMID 8872458.
- ↑ US 4371720, Johnson MR, Melvin LS, "2-Hydroxy-4-(substituted) phenyl cycloalkanes and derivatives.", issued 1 February 1983, assigned to Pfizer Inc.