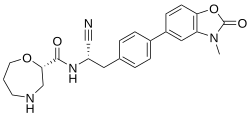
Brensocatib
 | |
| Clinical data | |
|---|---|
| Trade names | Brinsupri |
| Other names | AZD7986; INS1007 |
| AHFS/Drugs.com | Brinsupri |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Dipeptidyl peptidase 1 inhibitor |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H24N4O4 |
| Molar mass | 420.469 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Brensocatib, sold under the brand name Brinsupri, is a medication used for the treatment of bronchiectasis.[1] It is a dipeptidyl peptidase 1 (DPP1) inhibitor.[1][2] It is taken by mouth.[1]
Brensocatib was approved for medical use in the United States in August 2025.[1][3]
Medical uses
Brensocatib is indicated for the treatment of non-cystic fibrosis bronchiectasis in people aged twelve years of age and older.[1]
History
Bresocatib was discovered as a second generation DPP1 inhibitor, by scientists at AstraZeneca, eliminating aorta binding liabilities found with earlier compound series.[4] A phase III clinical trial, known as the ASPEN trial, was conducted to evaluate the safety and efficacy of brensocatib in patients with non-cystic fibrosis bronchiectasis.[5]
Society and culture
Legal status
Brensocatib was approved for medical use in the United States in August 2025.[6]
In October 2025, the Committee for Medicinal Products for Human Use of the European Medicines Agency adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Brinsupri, intended for the treatment of non-cystic fibrosis bronchiectasis in people aged twelve years of age and older.[7][8] The applicant for Brinsupri is Insmed Netherlands B.V.[9]
Names
Brensocatib is the international nonproprietary name.[10]
Brensocatib is sold under the brand name Brinsupri.[1]
References
- 1 2 3 4 5 6 7 https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/217673s000lbl.pdf
- ↑ Chalmers JD, Usansky H, Rubino CM, Teper A, Fernandez C, Zou J, et al. (October 2022). "Pharmacokinetic/Pharmacodynamic Evaluation of the Dipeptidyl Peptidase 1 Inhibitor Brensocatib for Non-cystic Fibrosis Bronchiectasis". Clinical Pharmacokinetics. 61 (10): 1457–1469. doi:10.1007/s40262-022-01147-w. PMC 9553789. PMID 35976570.
- ↑ "Novel Drug Approvals for 2025". U.S. Food and Drug Administration (FDA). 15 August 2025. Retrieved 17 August 2025.
- ↑ Doyle K, Lönn H, Käck H, Van de Poël A, Swallow S, Gardiner P, et al. (October 2015). "Discovery of Second Generation Reversible Covalent DPP1 Inhibitors Leading to an Oxazepane Amidoacetonitrile Based Clinical Candidate (AZD7986)". Journal of Medicinal Chemistry. 59 (20): 9457–9472. doi:10.1021/acs.jmedchem.6b01127. PMID 27690432.
- ↑ Chalmers JD, Burgel PR, Daley CL, De Soyza A, Haworth CS, Mauger D, et al. (April 2025). "Phase 3 Trial of the DPP-1 Inhibitor Brensocatib in Bronchiectasis". The New England Journal of Medicine. 392 (16): 1569–1581. doi:10.1056/NEJMoa2411664. PMID 40267423.
- ↑ "FDA Approves Brinsupri (brensocatib) as the First and Only Treatment for Non-Cystic Fibrosis Bronchiectasis, a Serious, Chronic Lung Disease" (Press release). Insmed. 12 August 2025. Retrieved 17 August 2025 – via PR Newswire.
- ↑ "Brinsupri EPAR". European Medicines Agency (EMA). 17 October 2025. Retrieved 27 October 2025. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "First treatment for serious chronic lung disease". European Medicines Agency (EMA) (Press release). 17 October 2025. Retrieved 27 October 2025.
- ↑ "Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 13-16 October 2025". European Medicines Agency (EMA). 17 October 2025. Retrieved 27 October 2025.
- ↑ World Health Organization (2020). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 83". WHO Drug Information. 34 (1). hdl:10665/339768.
External links
- Clinical trial number NCT04594369 for "A Study to Assess the Efficacy, Safety, and Tolerability of Brensocatib in Participants With Non-Cystic Fibrosis Bronchiectasis (ASPEN)" at ClinicalTrials.gov
- Clinical trial number NCT03218917 for "Assessment of INS1007 in Participants With Non-Cystic Fibrosis Bronchiectasis" at ClinicalTrials.gov