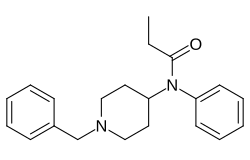
Benzylfentanyl
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N-(1-Benzylpiperidin-4-yl)-N-phenylpropanamide | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.014.559 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C21H26N2O |
| Molar mass | 322.452 g·mol−1 |
| Density | 1.113 g/cm3[1] |
| Boiling point | 502.2 °C (936.0 °F; 775.3 K)[1] |
| Vapor pressure | 3.36×10−8 mmHg[1] |
| Legal status | |
| Hazards | |
| GHS labelling:[4] | |
Pictograms |
 |
Signal word |
Danger |
Hazard statements |
H301, H412 |
Precautionary statements |
P264, P270, P273, P301+P316, P321, P330, P405, P501 |
| NFPA 704 (fire diamond) | |
| Flash point | 257.5 °C (495.5 °F; 530.6 K)[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
500 mg/kg (oral, as HCl salt, est.)[5] |
| Safety data sheet (SDS) | CATO Research Chemicals[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Benzylfentanyl (R-4129) is a fentanyl analog.[6] It was temporarily placed in the US Schedule I by emergency scheduling in 1985 due to concerns about its potential for abuse as a designer drug, but this placement was allowed to expire and benzylfentanyl was formally removed from controlled substance listing in 2010, after the DEA's testing determined it to be "essentially inactive" as an opioid.[7] It was added as a List I precursor instead in 2020 after it was determined that it was being used in fentanyl manufacture, which was previously thought to be beyond the technical capability of illicit labs.[3] Benzylfentanyl has a Ki of 213 nM at the mu opioid receptor, binding around 1/200 as strong as fentanyl itself, though it is still slightly more potent than codeine.[8][9]
See also
References
- 1 2 3 4 5 "Safety Data Sheet: N-(1-benzylpiperidin-4-yl)-N-phenylpropionamide". www.cato-chem.com. Guangzhou, CN: CATO Research Chemicals Inc. 8 August 2025. Retrieved 25 August 2025.
- ↑ "Anlage I BtMG - Einzelnorm". www.gesetze-im-internet.de (in German). Bundesamt fur Justiz. 2001. Retrieved 25 September 2025.
- 1 2 85 FR 20822
- ↑ "N-(1-Benzyl-4-piperidyl)-N-phenylpropanamide". PubChem. National Center for Biotechnology Information. Retrieved 25 September 2025.
- 1 2 "Cayman Chemical SDS (as hydrochloride)" (PDF). www.caymanchem.com. Cayman Chemicals. 28 August 2024. Retrieved 25 September 2025.
- ↑ Ruangyuttikarn, W.; Law, M.Y.; Rollins, D.E.; Moody, D.E. (1990). "Detection of fentanyl and its analogs by enzyme-linked immunosorbent assay". Journal of Analytical Toxicology. 14 (3): 160–4. doi:10.1093/jat/14.3.160. PMID 2374405.
- ↑ 75 FR 37300
- ↑ Alburges, Mario E. (June 1988). Utilization of a radioreceptor assay for the analysis of fentanyl analogs in urine (PDF) (PhD thesis). The University of Utah: Department of Pharmacology and Toxicology. Archived from the original (PDF) on 19 December 2013. Retrieved 25 September 2025.
- ↑ Chen, Z.R.; Irvine, R.J.; Somogyi, A.A.; Bochner, F. (1991). "Mu receptor binding of some commonly used opioids and their metabolites". Life Sci. 48 (22): 2165–71. doi:10.1016/0024-3205(91)90150-a. PMID 1851921.
