Avutometinib
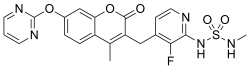 | |
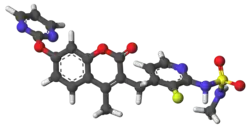 Skeletal formula and ball-and-stick model of avutometinib | |
| Clinical data | |
|---|---|
| Other names | RO-5126766; CH-5126766; CKI-27; R-7304; RG-7304 |
| Pharmacokinetic data | |
| Elimination half-life | 60 h (45.8–93.7 h) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C21H18FN5O5S |
| Molar mass | 471.46 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Avutometinib (INN; codenamed RO-5126766, CH-5126766, CKI-27, R-7304, RG-7304, and VS-6766 at various stages of its development) is an inhibitor of Ras-Raf-MEK-ERK signaling being developed as a potential treatment for cancer.
It was discovered by Chugai Pharmaceutical Co. (a subsidiary of Roche) through derivatization of a hit compound identified by high-throughput screening.[1] It was licensed by Verastem Oncology in 2020 for clinical trials.[2][3]
The co-packaged medication avutometinib/defactinib was approved for medical use in the United States in May 2025.[4][5]
References
- ↑ Ishii N, Harada N, Joseph EW, Ohara K, Miura T, Sakamoto H, Matsuda Y, Tomii Y, Tachibana-Kondo Y, Iikura H, Aoki T, Shimma N, Arisawa M, Sowa Y, Poulikakos PI, Rosen N, Aoki Y, Sakai T (July 2013). "Enhanced inhibition of ERK signaling by a novel allosteric MEK inhibitor, CH5126766, that suppresses feedback reactivation of RAF activity". Cancer Res. 73 (13): 4050–4060. doi:10.1158/0008-5472.CAN-12-3937. PMC 4115369. PMID 23667175.
- ↑ Adams, Ben (8 January 2020). "Verastem pens KRAS-focused drug licensing deal with Chugai". Fierce Biotech. Retrieved 5 June 2022.
- ↑ "Drug Profile: Avutometinib" (PDF). AdisInsight. 23 May 2022. Retrieved 5 June 2022.
- ↑ "FDA grants accelerated approval to the combination of avutometinib and defactinib for KRAS-mutated recurrent low-grade serous ovarian cancer". U.S. Food and Drug Administration. 8 May 2025. Archived from the original on 8 May 2025. Retrieved 16 May 2025.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "FDA Approves the Avmapki Fakzynja Combination Therapy as the First-Ever Treatment for Adult Patients with KRAS-mutated Recurrent Low-Grade Serous Ovarian Cancer". Verastem (Press release). Retrieved 16 May 2025.