Ampyrone
 | |
| Names | |
|---|---|
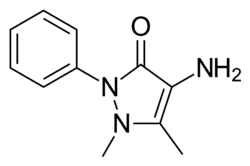
| Preferred IUPAC name
4-Amino-1,5-dimethyl-2-phenyl-3H-pyrazol-3-one[1] | |
| Other names
solvapyrin A, aminoazophene, aminoantipyrene, aminoantipyrine, metapyrazone | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.321 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C11H13N3O1[2] |
| Molar mass | 203.24 g/mol |
| Density | 1.207g/cm3 |
| Melting point | 106 to 110 °C (223 to 230 °F; 379 to 383 K) |
| Boiling point | 309 °C (588 °F; 582 K) @760mmHg |
| Hazards | |
| Flash point | 140.7 °C (285.3 °F; 413.8 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Ampyrone is a metabolite of aminopyrine with analgesic, anti-inflammatory, and antipyretic properties.[2] While the parent drug, aminopyrine, has been discouraged due to the risk of agranulocytosis,[3][4] ampyrone itself has significantly lower toxicity.[5] It is used as a reagent for biochemical reactions producing peroxides or phenols.[2] Ampyrone stimulates liver microsomes and is also used to measure extracellular water.[2]
By applying an ampyrone solution, scientists can make skin temporarily transparent (to red light); and e.g. see directly into the brain (of living young mice, when the skull is very thin, thus transparent, the solution doesn't have such an effect on it): "This opens a literal window to peek into the brain's development .. Not only can we image the structures of these neurons, but we can also image the neural activity over time in an animal model. In the future, this approach could enable us to look at how these circuits form during the development of an animal."[6][7]
References
- ↑ PubChem (25 March 2005). "4-Aminoantipyrine". PubChem. Retrieved 2022-05-09.
- 1 2 3 4 "4-Aminoantipyrine". pubchem.ncbi.nlm.nih.gov. 25 March 2005. Retrieved 2022-05-09.
- ↑ Bailey, D. N. (1983). "The unusual occurrence of 4-aminoantipyrine (4-aminophenazone) in human biological fluids". Journal of Analytical Toxicology. 7 (2): 76–78. doi:10.1093/jat/7.2.76. ISSN 0146-4760. PMID 6855207.
- ↑ PubChem. "Aminopyrine". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-08-26.
- ↑ PubChem. "4-Aminoantipyrine". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-08-26.
- ↑ "Researchers turn mouse scalp transparent to image brain development". news.stanford.edu. 2025-08-27. Retrieved 2025-08-28.
- ↑ Keck, Carl H. C.; Schmidt, Elizabeth L.; Roth, Richard H.; Floyd, Brendan M.; Tsai, Andy P.; Garcia, Hassler B.; Cui, Miao; Chen, Xiaoyu; Wang, Chonghe; Park, Andrew; Zhao, Su; Liao, Pinyu A.; Casey, Kerriann M.; Reineking, Wencke; Cai, Sa (2025-08-26). "Color-neutral and reversible tissue transparency enables longitudinal deep-tissue imaging in live mice". Proceedings of the National Academy of Sciences. 122 (35) e2504264122. doi:10.1073/pnas.2504264122.