Thermodynamics/Systems
Thermodynamic system
A thermodynamic system is a entity being subject to analysis. An entity is any collection of matter within a region of space.
Definition: Thermodynamic system
A thermodynamic system is a defined collection of entities subject to exchange of energy or matter, that is studied macroscopically. All space outside of this defined boundary is called the surroundings. A thermodynamic system can be decomposed into several subsystems, the entire composition of which can be called the total system.
There are two kinds of systems commonly used in analysis; closed systems and open systems. Very simply, closed systems are impermeable to mass transfer, while open systems are permeable to mass transfer. This definition does not restrict the flow or transfer of energy to or from these systems. Isolated systems are also useful to highlight when a system's boundary is impermeable both to mass transfer and any transfer of work or heat.
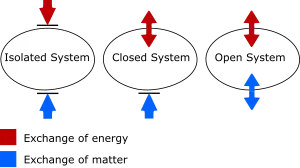
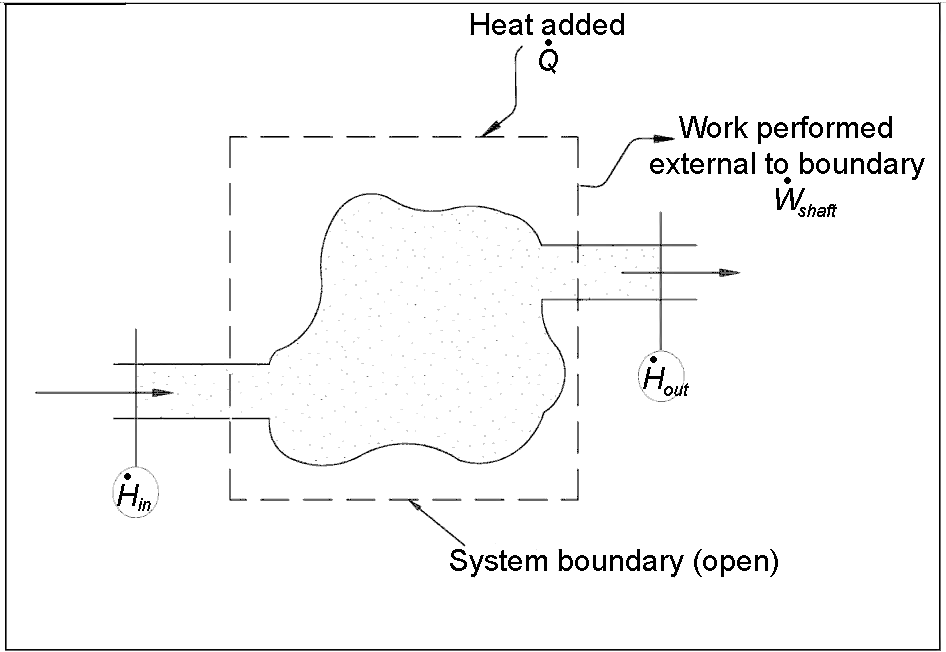
Open system
Definition: Open system
An open system is a system that exchange both energy and matter through its boundary.
System that comprises a fixed region in space where the system boundary is permeable to heat, work and mass transfer.
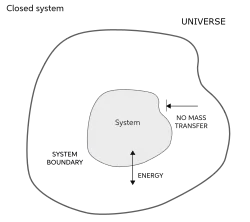
Closed system
Definition: Closed system
A closed system is one that can exchange energy but not matter through its boundary.
System that comprises a fixed quantity of mass where heat and work cross the system boundary, but where the boundary is impermeable to mass transfer.
Isolated system
Definition: Isolated system
An isolated system is one that can neither exchange energy nor matter through its boundary.
System boundaries
In order to better visualize and apply closed and open systems, it necessary to conceptualize the different way in which boundaries could be applied to analyze the same system.
Imagine a piston containing a compressible gas that is slowly being compressed. If time could be stopped, a boundary can be drawn inside the piston at a given point in time that describes the exact volume and mass of the gas. This boundary would contain every single gas particle, but perfectly exclude everything outside those particles. Now if time were allowed to continue, and the piston continued to compress, the volume inside the piston would decrease by some elementary amount, and assuming the piston is perfectly sealed, the mass of the gas contained inside the piston would remain constant.
To-do: add GIF or diagram or series of pictures describing the paragraph above.