Tezacaftor
 | |
| Names | |
|---|---|
| Other names | VX-661 |
IUPAC name
| |
| Legal | |
| License data |
|
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ATC code | |
| Chemical and physical data | |
| Formula | C26H27F3N2O6 |
| Molar mass | 520.505 g·mol−1 |
InChI
| |
Tezacaftor is a medication used for the treatment of cystic fibrosis.[1][2] It is available in fixed-dose combination medications.[3][4][5]
The combination of tezacaftor with ivacaftor (brand name Symdeko) was approved for medical use in the United States in February 2018,[6][1] and in Canada in June 2018.[7] As brand name Symkevi it was approved for medical use in the European Union in October 2018.[8]
The combination of tezacaftor with elexacaftor and ivacaftor (brand name Trikafta) was approved for medical use in the United States in October 2019,[9][10][11] and in Canada in June 2021.[12] As brand name Kaftrio it was approved for medical use in the European Union in August 2020.[13]
The combination of tezacaftor with vanzacaftor and deutivacaftor (brand name Alyftrek) was approved for medical use in the United States in December 2024.[14]
Medical use
Tezacaftor is a medication used in the treatment of cystic fibrosis , a genetic condition that affects the lungs and other organs. It works as a cystic fibrosis transmembrane conductance regulator modulator, helping to improve the function of defective CFTR proteins caused by certain genetic mutations, such as F508del[15]
Mechanism of action
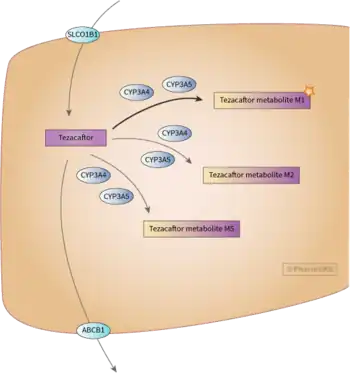
Tezacaftor acts as a corrector to help the folding and presentation of the CFTR protein to the cell surface, which improves its function for individuals with a F508del mutation.[1][17][18]
Clinical trials
The EVOLVE and EXPAND study findings were published in 2017.[19]
EVOLVE trial
The EVOLVE trial analyzed tezacaftor/ivacaftor in participants with cystic fibrosis, specifically with the homozygous for Phe508del mutation.[20] The EVOLVE trial is a phase 3, double-blinded, multicenter, randomized, placebo-controlled, parallel-group trial, that was which evaluated therapy with a combination of tezacaftor and ivacaftor in participants that are aged 12 years of age and older.[20]
510 participants were randomized and 509 participants were given either 100 mg of tezacaftor once daily and 150 mg of ivacaftor twice daily or a placebo for 24 weeks.[20] The combination of drugs was efficacious in participants who had cystic fibrosis with the Phe508del mutation and the adverse effects in both treatment groups were similar.[20]
History
The US Food and Drug Administration (FDA) granted the application for tezacaftor and ivacaftor combination therapy orphan drug and priority review designations, and granted the approval of Symdeko to Vertex Pharmaceuticals.[2][21] The European Medicines Agency (EMA) designated the combination an orphan medicine.[8]
The FDA and the EMA granted the application for elexacaftor, tezacaftor, and ivacaftor combination therapy an orphan drug designation.[22][13][23]
The FDA granted the application for vanzacaftor, tezacaftor, and deutivacaftor combination therapy an orphan drug designation.[24]
Society and culture
Names
Tezacaftor is the international nonproprietary name.[25]
References
- ↑ 1.0 1.1 1.2 "Drug Trials Snapshots: Symdeko". U.S. Food and Drug Administration (FDA). 7 March 2018. Archived from the original on 21 November 2019. Retrieved 20 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 2.0 2.1 "FDA expands approval of treatment for cystic fibrosis to include patients ages 6 and older". U.S. Food and Drug Administration (FDA) (Press release). 21 June 2019. Archived from the original on 21 November 2019. Retrieved 20 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Lommatzsch ST, Taylor-Cousar JL (2019). "The combination of tezacaftor and ivacaftor in the treatment of patients with cystic fibrosis: clinical evidence and future prospects in cystic fibrosis therapy". Therapeutic Advances in Respiratory Disease. 13: 1753466619844424. doi:10.1177/1753466619844424. PMC 6487765. PMID 31027466.
- ↑ Paterson SL, Barry PJ, Horsley AR (January 2020). "Tezacaftor and ivacaftor for the treatment of cystic fibrosis". Expert Review of Respiratory Medicine. 14 (1): 15–30. doi:10.1080/17476348.2020.1682998. PMID 31626570. S2CID 204787700.
- ↑ Guerra L, Favia M, Di Gioia S, Laselva O, Bisogno A, Casavola V, Colombo C, Conese M (August 2020). "The preclinical discovery and development of the combination of ivacaftor + tezacaftor used to treat cystic fibrosis". Expert Opinion on Drug Discovery. 15 (8): 873–891. doi:10.1080/17460441.2020.1750592. hdl:11586/295256. PMID 32290721. S2CID 215773568.
- ↑ "Symdeko- tezacaftor and ivacaftor kit". DailyMed. 9 February 2024. Archived from the original on 21 July 2020. Retrieved 24 December 2024.
- ↑ "Symdeko Product information". Health Canada. Archived from the original on 1 June 2022. Retrieved 31 May 2022.
- ↑ 8.0 8.1 "Symkevi EPAR". European Medicines Agency (EMA). 27 February 2017. Archived from the original on 8 January 2021. Retrieved 24 December 2024.
- ↑ "Trikafta- elexacaftor, tezacaftor, and ivacaftor kit". DailyMed. 29 January 2020. Archived from the original on 21 July 2020. Retrieved 22 August 2020.
- ↑ "Drug Trials Snapshots: Trikafta". U.S. Food and Drug Administration (FDA). 31 October 2019. Archived from the original on 20 November 2019. Retrieved 24 December 2024.
- ↑ "FDA approves new breakthrough therapy for cystic fibrosis". U.S. Food and Drug Administration (FDA) (Press release). 21 October 2019. Archived from the original on 13 November 2019. Retrieved 13 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Trikafta Product information". Health Canada. 22 June 2021. Archived from the original on 25 September 2023. Retrieved 24 December 2024.
- ↑ 13.0 13.1 "Kaftrio EPAR". European Medicines Agency (EMA). 14 December 2018. Archived from the original on 20 September 2020. Retrieved 24 December 2024.
- ↑ "Vertex Announces US FDA Approval of Alyftrek, a Once-Daily Next-in-Class CFTR Modulator for the Treatment of Cystic Fibrosis" (Press release). Vertex. 20 December 2024. Archived from the original on 26 December 2024. Retrieved 24 December 2024 – via Business Wire.
- ↑ "Tezacaftor". pubchem.ncbi.nlm.nih.gov. Archived from the original on 7 April 2025. Retrieved 20 March 2025.
- ↑ "Tezacaftor". PharmGKB.
- ↑ "Tezacaftor (VX-661) for Cystic Fibrosis". Cystic Fibrosis News Today. Pensacola, FL: BioNews Services, LLC. Archived from the original on 29 September 2018. Retrieved 18 March 2025.
- ↑ Ridley, Kaden (2020). "Elexacaftor-Tezacaftor-Ivacaftor: The First Triple-Combination Cystic Fibrosis Transmembrane Conductance Regulator Modulating Therapy". The Journal of Pediatric Pharmacology and Therapeutics. 25 (3): 192–197. doi:10.5863/1551-6776-25.3.192. PMC 7134581. PMID 32265602.
- ↑ Boyles S (13 February 2018). "FDA Approves New Cystic Fibrosis Drug Combo - Symdeko approved for patients with specific CFTR mutations". MedPage Today, LLC. Archived from the original on 4 February 2023. Retrieved 18 March 2025.
- ↑ 20.0 20.1 20.2 20.3 Taylor-Cousar, Jennifer (23 November 2017). "Tezacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del". New England Journal of Medicine. 377 (21): 2013–2023. doi:10.1056/NEJMoa1709846. PMID 29099344. S2CID 205102514.
- ↑ "Tezacaftor and Ivacaftor Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 15 June 2017. Archived from the original on 29 October 2020. Retrieved 25 October 2020.
- ↑ "Trikafta Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 29 August 2018. Archived from the original on 8 September 2024. Retrieved 24 December 2024.
- ↑ "EU/3/18/2116 - orphan designation for treatment of cystic fibrosis". European Medicines Agency (EMA). 14 December 2018. Archived from the original on 18 May 2024. Retrieved 24 December 2024.
- ↑ "Alyftrek Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). Archived from the original on 21 December 2023. Retrieved 24 December 2024.
- ↑ World Health Organization (2016). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 76". WHO Drug Information. 30 (3). hdl:10665/331020.