Pasireotide
 | |
| Names | |
|---|---|
| Trade names | Signifor, Signifor LAR |
| Other names | SOM230 |
IUPAC name
| |
| Clinical data | |
| Drug class | Somatostatin analog[1] |
| Main uses | Cushing's disease, acromegaly[1] |
| Side effects | High blood sugar, diarrhea, abdominal pain, nausea, gallstones, injection site reactions, tiredness[2] |
| Pregnancy category |
|
| Routes of use | Subcutaneous injection, intramuscular injection |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
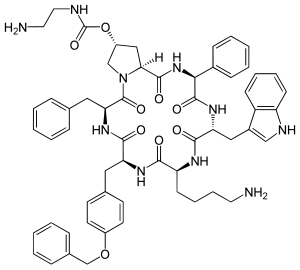
| Formula | C58H66N10O9 |
| Molar mass | 1047.227 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Pasireotide, sold under the brand name Signifor, is a medication used to treat Cushing's disease and acromegaly.[1] It is used in Cushing's disease when surgery is not effective, and in acromegaly when neither surgery nor another somatostatin is effective.[1] It is given by injection under the skin or into a muscle.[1]
Common side effects include high blood sugar, diarrhea, abdominal pain, nausea, gallstones, injection site reactions, and tiredness.[2] Other side effects may include low cortisol, slow heart rate, and liver problems.[3] It is a somatostatin analog which blocks the release of growth hormone and cortisol.[2]
Pasireotide was approved in Europe and the United States in 2012.[2][3] In the United Kingdom it costs the NHS about £2,300 to £3,200 a month as of 2021.[1] In the United States this amount costs about 15,300 USD.[4]
Medical uses
It is used in people who fail or are ineligible for surgical therapy.[5][6][7] A long-acting formulation is used for acromegaly.[8] [2][9]
Dosage
For Cushing's it is used at a dose of 600 mcg injected under the skin twice per day; which may be increased to 900 mcg twice per day.[1] A long acting formulation into a muscle every 4 weeks at a dose of 10 to 40 mg may also be used.[1]
For acromegaly injections of 40 to 60 mg into a muscle every 4 weeks may be used.[1]
History
It is an orphan drug approved in the United States[10] and the European Union.[2][11]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 995. ISBN 978-0857114105.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Signifor EPAR". European Medicines Agency (EMA). Archived from the original on 12 November 2020. Retrieved 13 May 2020. Archived 12 November 2020 at the Wayback Machine Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 3.0 3.1 "Pasireotide Monograph for Professionals". Drugs.com. Archived from the original on 21 August 2021. Retrieved 26 October 2021. Archived 21 August 2021 at the Wayback Machine
- ↑ "Signifor Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 26 October 2021.
- ↑ "Pasireotide Orphan Drug Designation and Approval". U.S. Food and Drug Administration (FDA). 24 December 1999. Archived from the original on 21 August 2021. Retrieved 13 May 2020. Archived 21 August 2021 at the Wayback Machine
- ↑ "EU/3/09/671". European Medicines Agency. 17 September 2018. Archived from the original on 8 January 2021. Retrieved 13 May 2020. Archived 8 January 2021 at the Wayback Machine Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ Mancini T, Porcelli T, Giustina A (October 2010). "Treatment of Cushing disease: overview and recent findings". Therapeutics and Clinical Risk Management. 6: 505–16. doi:10.2147/TCRM.S12952. PMC 2963160. PMID 21063461.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ↑ "Signifor LAR (pasireotide) for injectable suspension". U.S. Food and Drug Administration (FDA). 1 March 2016. Archived from the original on 31 October 2020. Retrieved 13 May 2020. Archived 31 October 2020 at the Wayback Machine
- ↑ Tucker ME (17 December 2014). "FDA Approves Pasireotide for Treating Acromegaly". Medscape. Archived from the original on 27 August 2015. Retrieved 2 August 2015. Archived 27 August 2015 at the Wayback Machine
- ↑ "Drug Approval Package: Signifor (pasireotide) Injection NDA #200677". U.S. Food and Drug Administration (FDA). 24 December 1999. Archived from the original on 9 April 2021. Retrieved 13 May 2020. Archived 9 April 2021 at the Wayback Machine
- ↑ "Summary of Product Characteristics: Signifor" (PDF). European Medicines Agency. Archived (PDF) from the original on 2018-06-14. Retrieved 2021-08-24. Archived 2018-06-14 at the Wayback Machine
External links
| External sites: |
|
|---|---|
| Identifiers: |