Patisiran
 | |
| Names | |
|---|---|
| Trade names | Onpattro |
| Other names | ALN-18328 |
| Clinical data | |
| Drug class | Small interfering RNA (siRNA)[1] |
| Main uses | Polyneuropathy in hereditary transthyretin-mediated amyloidosis[1] |
| Side effects | Upper respiratory infection, shortness of breath, muscle spasms, joint pain, redness, dizziness[1] |
| Pregnancy category |
|
| Routes of use | Intravenous |
| Typical dose | 300 mcg/kg q 21 days[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status |
|
| Chemical and physical data | |
| Formula | C412H520N148O290P40 |
| Molar mass | 13424.388 g·mol−1 |
Patisiran, sold under the brand name Onpattro, is a medication used to treat polyneuropathy in people with hereditary transthyretin-mediated amyloidosis.[1] It is given by gradual injection into a vein.[2]
Common side effects include upper respiratory infection, shortness of breath, muscle spasms, joint pain, redness, and dizziness.[1] Other side effects may include vitamin A deficiency.[1] Safety in pregnancy is unclear; though evidence shows harm in other animals.[1] It is a small piece of RNA that blocks the production of abnormal transthyretin.[3]
Patisiran was approved for medical use in Europe and the United States in 2018.[3][1] In the United Kingdom a single vial of 10 mg costs the NHS about £7,700 as of 2021.[2] This amount in the United States costs about 10,000 USD.[4]
Medical uses
Dosage
It is given at a dose of 300 mcg/kg every 21 days.[2] The maximum dose is 30 mg.[2]
Mechanism of action
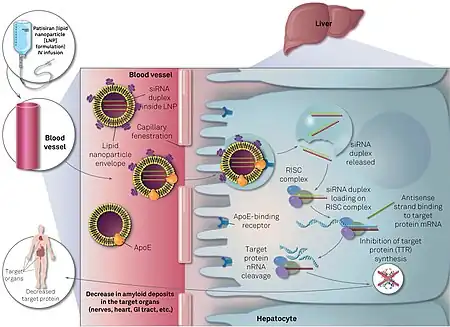
It is a gene silencing drug that interferes with the production of an abnormal form of transthyretin.[6]
It utilizes a novel approach to target and reduce production of the TTR protein in the liver via the RNAi pathway.[7]
History
Patisiran was granted orphan drug status, fast track designation, priority review and breakthrough therapy designation due to its novel mechanism and the rarity of the condition it treats.[8][9]
It was approved for medical use in the United States and in the European Union in August 2018.[10][11]
It is the first small interfering RNA-based drug approved by the U.S. Food and Drug Administration (FDA) and the first drug approved by the FDA to treat this condition.[12]
The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[13]
Society and culture
Economics
Patisiran was developed and is marketed by Alnylam.[14]
The per-patient cost is between US$451,430 and US$677,145 per year, depending on the number of vials needed.[15][16][17]
As of 2020, there were 1050 patients globally receiving the medication generating $65.5M in net-revenues for Alnylam Pharmaceuticals.[18][19]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Patisiran Monograph for Professionals". Drugs.com. Archived from the original on 26 January 2021. Retrieved 26 October 2021. Archived 26 January 2021 at the Wayback Machine
- ↑ 2.0 2.1 2.2 2.3 2.4 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1109. ISBN 978-0857114105.
- ↑ 3.0 3.1 "Onpattro". Archived from the original on 8 November 2020. Retrieved 26 October 2021. Archived 8 November 2020 at the Wayback Machine
- ↑ "Onpattro Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 24 June 2021. Retrieved 26 October 2021. Archived 24 June 2021 at the Wayback Machine
- ↑ Pinto, Marcus Vinicius; Barreira, Amilton Antunes; Bulle, Acary Souza; Freitas, Marcos Raimundo Gomes de; França, Marcondes Cavalcante; Gondim, Francisco de Assis Aquino; Marrone, Carlo Domenico; Marques, Wilson; Nascimento, Osvaldo J. M.; Rotta, Francisco Tellechea; Pupe, Camila; Waddington-Cruz, Márcia (September 2018). "Brazilian consensus for diagnosis, management and treatment of transthyretin familial amyloid polyneuropathy". Arquivos De Neuro-Psiquiatria. 76 (9): 609–621. doi:10.1590/0004-282X20180094. ISSN 1678-4227.
- ↑ Kristen, Arnt V; Ajroud-Driss, Senda; Conceição, Isabel; Gorevic, Peter; Kyriakides, Theodoros; Obici, Laura (2018-11-27). "Patisiran, an RNAi therapeutic for the treatment of hereditary transthyretin-mediated amyloidosis". Neurodegenerative Disease Management. 9 (1): 5–23. doi:10.2217/nmt-2018-0033. ISSN 1758-2024. PMID 30480471.
- ↑ "Onpattro (patisiran)". www.centerwatch.com. Archived from the original on 2021-06-24. Retrieved 2021-06-18. Archived 2021-06-24 at the Wayback Machine
- ↑ "FDA approves first-of-its kind targeted RNA-based therapy to treat a rare disease" (Press release). U.S. Food and Drug Administration (FDA). 10 August 2018. Archived from the original on 7 September 2018. Retrieved 11 August 2018.
- ↑ Brooks M (10 August 2018). "FDA OKs Patisiran (Onpattro) for Polyneuropathy in hAATR". Medscape. WebMD. Archived from the original on 4 July 2019. Retrieved 10 August 2018. Archived 4 July 2019 at the Wayback Machine
- ↑ "Drug Approval Package: Onpattro (patisiran)". U.S. Food and Drug Administration (FDA). 7 September 2018. Archived from the original on 12 April 2021. Retrieved 2 September 2020. Archived 12 April 2021 at the Wayback Machine
- ↑ "Onpattro EPAR". European Medicines Agency (EMA). Archived from the original on 8 November 2020. Retrieved 2 September 2020. Archived 8 November 2020 at the Wayback Machine
- ↑ Loftus P (10 August 2018). "New Kind of Drug, Silencing Genes, Gets FDA Approval". The Wall Street Journal. Archived from the original on 10 August 2018. Retrieved 10 August 2018. Archived 10 August 2018 at the Wayback Machine
- ↑ New Drug Therapy Approvals 2018 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2019. Archived from the original on 17 September 2020. Retrieved 16 September 2020.
- ↑ Reidy, Chris (October 22, 2012). "Alnylam, Genzyme Form Alliance". The Boston Globe. Archived from the original on 7 May 2021. Retrieved 5 May 2021. Archived 7 May 2021 at the Wayback Machine
- ↑ Information, National Center for Biotechnology; Pike, U. S. National Library of Medicine 8600 Rockville; MD, Bethesda (2019-08-01). Executive Summary. Canadian Agency for Drugs and Technologies in Health. Archived from the original on 2021-10-20. Retrieved 2021-06-24.
{{cite book}}: CS1 maint: numeric names: authors list (link) Archived 2021-10-20 at the Wayback Machine - ↑ Lipschultz B, Cortez M (10 August 2018). "Rare-Disease Treatment From Alnylam to Cost $450,000 a Year". Bloomberg. Archived from the original on 14 November 2019. Retrieved 11 August 2018. Archived 14 November 2019 at the Wayback Machine
- ↑ "Onpattro Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 2021-06-24. Retrieved 2021-06-23. Archived 2021-06-24 at the Wayback Machine
- ↑ "Patisiran and Vutrisiran, in development for the Treatment of Transthyretin-Mediated Amyloidosis" (PDF). Alnylam Pharmaceuticals. Archived (PDF) from the original on 2021-10-09. Archived 2021-10-09 at the Wayback Machine
- ↑ "Alnylam Pharmaceuticals Reports Fourth Quarter and Full Year 2020 Financial Results and Highlights Recent Period Activity". www.businesswire.com. 2021-02-11. Archived from the original on 2021-06-24. Retrieved 2021-06-23. Archived 2021-06-24 at the Wayback Machine
External links
| External sites: |
|
|---|---|
| Identifiers: |