Mavacamten
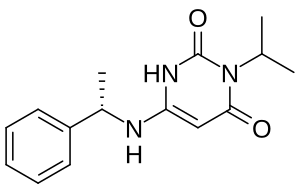 | |
| Names | |
|---|---|
| Trade names | Camzyos |
| Other names | MYK-461 |
IUPAC name
| |
| Clinical data | |
| Drug class | Cardiac myosin inhibitor |
| Main uses | Hypertrophic obstructive cardiomyopathy (HCOM)[1] |
| Side effects | Dizziness, syncope[1] |
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C15H19N3O2 |
| Molar mass | 273.336 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Mavacamten, sold under the brand name Camzyos, is a medication used to treat hypertrophic obstructive cardiomyopathy (HCOM).[1] It is taken by mouth.[1]
Common side effects include dizziness and syncope.[1] Other side effects may include heart failure.[1] Use in pregnancy may harm the baby.[1] It is a cardiac myosin inhibitor.[1]
Mavacamten was approved for medical use in the United States in 2022.[1] It is not available in Europe or the United Kingdom as of 2022.[3] In the United States a month of medication costs about 7,800 USD.[4]
Medical uses
Mavacamten is indicated for the treatment of adults with symptomatic New York Heart Association class II-III obstructive hypertrophic cardiomyopathy to improve functional capacity and symptoms.[1]
Dosage
It is started at a dose of 5 mg once per day, with long term doses between 2.5 and 15 mg once per day.[1]
History
Mavacamten was granted orphan drug designation by the U.S. Food and Drug Administration (FDA).[5] It was developed by the MyoKardia, a subsidiary of Bristol Myers Squibb.[6]
Society and culture
Names
Mavacamten is the international nonproprietary name (INN).[7]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 "Camzyos- mavacamten capsule, gelatin coated". DailyMed. 28 April 2022. Archived from the original on 3 July 2022. Retrieved 15 May 2022. Archived 3 July 2022 at the Wayback Machine
- ↑ "Archive copy". Archived from the original on 2022-11-13. Retrieved 2022-11-29.
{{cite web}}: CS1 maint: archived copy as title (link) Archived 2022-11-13 at the Wayback Machine - ↑ "Mavacamten". SPS - Specialist Pharmacy Service. 19 October 2018. Archived from the original on 24 June 2022. Retrieved 12 December 2022. Archived 24 June 2022 at the Wayback Machine
- ↑ "Camzyos Prices, Coupons, Copay & Patient Assistance". Drugs.com. Archived from the original on 22 May 2023. Retrieved 12 December 2022. Archived 22 May 2023 at the Wayback Machine
- ↑ "Mavacamten Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 27 April 2016. Archived from the original on 3 July 2022. Retrieved 29 April 2022. Archived 3 July 2022 at the Wayback Machine
- ↑ "Bristol Myers Squibb Completes Acquisition of MyoKardia, Strengthening Company's Leading Cardiovascular Franchise". Business Wire. 17 November 2020. Archived from the original on 29 April 2022. Retrieved 29 April 2022. Archived 29 April 2022 at the Wayback Machine
- ↑ World Health Organization (2017). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 78". WHO Drug Information. 31 (3). hdl:10665/330961.
Further reading
- Xie J, Wang Y, Xu Y, Fine JT, Lam J, Garrison LP (2022). "Assessing health-related quality-of-life in patients with symptomatic obstructive hypertrophic cardiomyopathy: EQ-5D-based utilities in the EXPLORER-HCM trial". Journal of Medical Economics. 25 (1): 51–58. doi:10.1080/13696998.2021.2011301. PMID 34907813.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- Clinical trial number NCT03470545 for "Clinical Study to Evaluate Mavacamten (MYK-461) in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy (EXPLORER-HCM)" at ClinicalTrials.gov