Eflapegrastim
| Names | |
|---|---|
| Trade names | Rolvedon |
| Other names | Eflapegrastim-xnst, HM-10460A, SPI-2012 |
| Clinical data | |
| Drug class | Granulocyte colony-stimulating factor (G-CSF)[1] |
| Main uses | Reduce the risk of febrile neutropenia with chemotherapy[1] |
| Side effects | Tiredness, nausea, diarrhea, pain, fever, rash, low red blood cells[1] |
| Routes of use | Subcutaneous |
| Typical dose | 13.2 mg[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status | |
Eflapegrastim, sold under the brand names Rolvedon, is a medication used to reduce the risk of febrile neutropenia in non-myeloid cancers receiving chemotherapy that results in bone marrow suppression.[1] It is given by injection under the skin.[1] It is given about 24 hours after chemotherapy.[1]
Common side effects include tiredness, nausea, diarrhea, pain, fever, rash, and low red cells.[1] Other side effects may include spleen rupture, ARDS, anaphylaxis, low platelets, glomerulonephritis, and myelodysplastic syndrome.[1] It is a granulocyte colony-stimulating factor (G-CSF), specifically a leukocyte growth factor.[1]
Eflapegrastim was approved for medical use in the United States in 2022.[1] It is not approved in Europe or the United Kingdom as of 2022.[2] It costs about 4,700 USD per dose in the United States as of 2022.[3]
Medical uses
Eflapegrastim is indicated to decrease the incidence of infection, as manifested by febrile neutropenia, in adults with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with clinically significant incidence of febrile neutropenia.[1]
Its efficacy has been shown to be non-inferior to pegfilgrastim.[1]
Dosage
It is generally given at a dose of 13.2 mg once per chemotherapy cycle.[1]
Mechanism of action
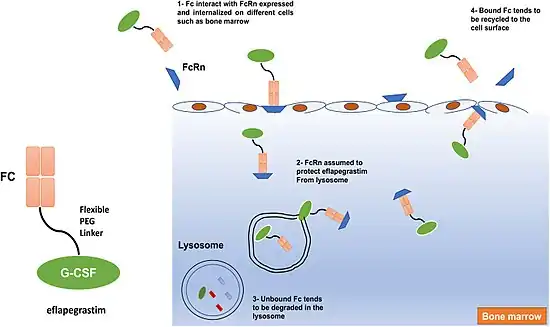
In terms of the mode of action for Eflapegrastim we find that it is a rhG-CSF, which binds to G-CSF receptors on myeloid progenitor cells,as well as neutrophils. This in turn causes signaling pathways to adjust neutrophil differentiation, and survival, which ultimately sees more white blood cell production[4]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 "Rolvedon- eflapegrastim-xnst injection, solution". DailyMed. 23 September 2022. Archived from the original on 16 October 2022. Retrieved 16 October 2022.
- ↑ "Eflapegrastim". SPS - Specialist Pharmacy Service. 16 March 2018. Archived from the original on 25 June 2022. Retrieved 13 December 2022.
- ↑ "Rolvedon Prices, Coupons, Copay & Patient Assistance". Drugs.com. Retrieved 13 December 2022.
- ↑ 4.0 4.1 Theyab, Abdulrahman; Alsharif, Khalaf F.; Alzahrani, Khalid J.; Oyouni, Atif Abdulwahab A.; Hawsawi, Yousef MohammedRabaa; Algahtani, Mohammad; Alghamdi, Saad; Alshammary, Amal F. (5 January 2023). "New insight into strategies used to develop long-acting G-CSF biologics for neutropenia therapy". Frontiers in Oncology. 12. doi:10.3389/fonc.2022.1026377.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
External links
| External sites: |
|
|---|---|
| Identifiers: |
- Clinical trial number NCT02643420 for "SPI-2012 vs Pegfilgrastim in the Management of Neutropenia in Participants With Breast Cancer With Docetaxel and Cyclophosphamide (ADVANCE) (ADVANCE)" at ClinicalTrials.gov
- Clinical trial number NCT02953340 for "SPI-2012 vs Pegfilgrastim in Management of Neutropenia in Breast Cancer Participants With Docetaxel and Cyclophosphamide" at ClinicalTrials.gov