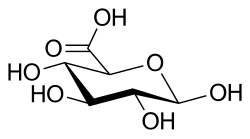
Glucuronic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
β-D-Glucopyranuronic acid | |
| Systematic IUPAC name
(2S,3S,4S,5R,6R)-3,4,5,6-Tetrahydroxyoxane-2-carboxylic acid | |
| Other names
β-D-Glucuronic acid, GlcA | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.026.807 |
| KEGG | |
| MeSH | Glucuronic+acid |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C6H10O7 |
| Molar mass | 194.139 g·mol−1 |
| Melting point | 159 to 161 °C (318 to 322 °F; 432 to 434 K)[1] |
| Related compounds | |
Related uronic acids |
Alluronic acid, Altruronic acid, Arabinuronic acid, Fructuronic acid, Galacturonic acid, Guluronic acid, Iduronic acid, Lyxuronic acid, Mannuronic acid, Psicuronic acid, Riburonic acid, Ribuluronic acid, Sorburonic acid, Tagaturonic acid, Taluronic acid, Xyluluronic acid, Xyluronic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Glucuronic acid (GCA, from Ancient Greek: γλεῦκος + οὖρον, lit. 'sweet wine, must + urine') is a uronic acid that was first isolated from urine (hence the name "uronic acid"). It is found in many gums such as gum arabic (approx. 18%), xanthan, and kombucha tea and is important for the metabolism of microorganisms, plants and animals.
Properties
Glucuronic acid is a sugar acid derived from glucose[2]
Sodium glucuronate can be produced by the direct oxidation of starch with concentrated nitric acid. In this preparation the low availability of water keeps the starch polymers from hydrolyzing and oxidizes only the free hydroxyls, in much the same way that nitrogen dioxide would oxidize the starch. Once this reaction is complete and the starch/nitric acid mix turns clear (after giving off nitrogen dioxide gas), the solution can be diluted, and hydrolyzed with another mineral acid. Then the oxidation is slowly quenched with sodium hydroxide (or sodium bicarbonate), forming sodium glucuronate, which can be crystallized out of solution. With transition metals, it forms complexes such as iron(III) glucuronate, iron(II) glucuronate, and copper(II) glucuronate.
Functions
Glucuronidation
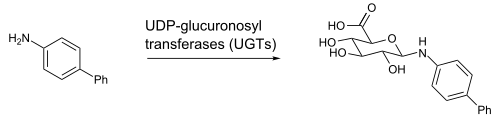
It is possible to exhaust the body's supply of glucuronic acid by combining multiple drugs/substances whose metabolism and excretion are dependent on glucuronidation. Although most such substances have secondary metabolic routes which become prominent following GCA depletion, the rate of metabolism is reduced enough to produce a marked accumulation of all GCA substrates in the system; this often increases drug concentrations in the blood by medically relevant amounts. In the most severe cases, permanent and debilitating organ damage (particularly of the liver, kidneys, heart, and brain), and even death, have been known to occur. Ethanol, morphine, paracetamol (acetaminophen), cyclooxygenase inhibitors (NSAIDs), endogenous steroids, and certain benzodiazepines are all capable of contributing to GCA depletion, with ethanol and acetaminophen being the most commonly implicated substances involved in cases of accidental overdoses which have been positively attributed to glucuronic acid depletion.
Excessive quantities of GCA can also be hazardous to health. Tobacco smoke, most barbiturates, and some carbamates are known to stimulate GCA production. Increased GCA activity results in a decrease of the concentration and metabolic half-life of glucuronic acid substrates, causing the plasma levels of glucuronidated drugs to fall below their therapeutic threshold. Excessive glucuronidation of the substrates may result in an inadequate response to traditional doses of affected medications and, unless the drug has a very wide therapeutic index, will generally result in the acute failure of the pharmacotherapy and necessitate the transition from one or more implicated drugs to an equivalent regimen of non-glucuronidated alternatives. A select number of antidepressants and a wide range of anti-psychotic agents are glucuronidation ligands, but due to their delayed mechanism of action and pharmacokinetic properties the decrease of their plasma concentrations may not be immediately apparent and tends to present as a sudden and intense relapse of symptoms instead of a gradual regression to the behaviors and thought patterns exhibited by the patient prior to the initiation of their pharmacological treatment.
Role in disease
Glucuronic acid, as well as the glucuronidated metabolite of ethanol, ethyl glucuronide (ETG), acts on toll-like receptor 4 to aggravate both acute and chronic inflammatory conditions and increases the perceived severity of pain in patients with chronic pain conditions, via up-regulation of the production and release of endogenous inflammatory signaling molecules within the body. Long-term agonism of the TLR4 receptor (such as that which occurs from GCA, ETG, and opiates) results in chronically painful conditions being perceived as considerably more severe than they did previously, while pre-existing, tolerable yet occasionally painful activities can become more painful than before and will begin to be aggravated by briefer and less physically demanding activities. It also can cause equally painful responses to decreasingly noxious (irritating) stimuli, eventually resulting in considerable agony from stimuli which would not cause any amount of pain to most individuals.[3]
Use
The Glucuronidated metabolites of various chemicals can be tested for in bodily fluids.[4] Ethyl glucuronide and ethyl sulfate are excreted in urine as metabolites of ethanol and are used to monitor alcohol use.[5]
Glucuronic acid and gluconic acid are fermentation products in Kombucha tea.[6]
Glucuronic acid is a precursor of ascorbic acid (vitamin C, formerly called L-hexuronic acid).[7]
See also
- Gluconic acid
- Isosaccharinic acid
- Uronic acid
References
- ↑ D-Glucuronic acid at Sigma-Aldrich
- ↑ Ho A, Sinick J, Esko T, Fischer K, Menni C, Zierer J, Matey-Hernandez M, Fortney K, Morgen EK (2019-09-26). "Circulating glucuronic acid predicts healthspan and longevity in humans and mice". Aging. 11 (18): 7694–7706. doi:10.18632/aging.102281. ISSN 1945-4589. PMC 6781977. PMID 31557729.
- ↑ Lewis SS, Hutchinson MR, Zhang Y, Hund DK, Maier SF, Rice KC, Watkins LR (2013). "Glucuronic acid and the ethanol metabolite ethyl-glucuronide cause toll-like receptor 4 activation and enhanced pain". Brain, Behavior, and Immunity. 30: 24–32. doi:10.1016/j.bbi.2013.01.005. PMC 3641160. PMID 23348028.
- ↑ Yang G, Ge S, Singh R, Basu S, Shatzer K, Zen M, Liu J, Tu Y, Zhang C, Wei J, Shi J, Zhu L, Liu Z, Wang Y, Gao S (2017-04-03). "Glucuronidation: driving factors and their impact on glucuronide disposition". Drug Metabolism Reviews. 49 (2): 105–138. doi:10.1080/03602532.2017.1293682. ISSN 0360-2532. PMC 7660525. PMID 28266877.
- ↑ Jatlow PI, Agro A, Wu R, Nadim H, Toll BA, Ralevski E, Nogueira C, Shi J, Dziura JD, Petrakis IL, O'Malley SS (2014-04-28). "Ethyl Glucuronide and Ethyl Sulfate Assays in Clinical Trials, Interpretation, and Limitations: Results of a Dose Ranging Alcohol Challenge Study and 2 Clinical Trials". Alcoholism: Clinical and Experimental Research. 38 (7): 2056–2065. doi:10.1111/acer.12407. PMC 4107122. PMID 24773137.
- ↑ Blanc P (February 1996). "Characterization of the tea fungus metabolites". Biotechnology Letters. 18 (2): 139–142. doi:10.1007/BF00128667. S2CID 34822312.
- ↑ Gerhard Michal, Dietmar Schomburg (2012), Biochemical Pathways: An Atlas of Biochemistry and Molecular Biology (2nd ed.), Wiley, p. 145a, ISBN 978-0-470-14684-2